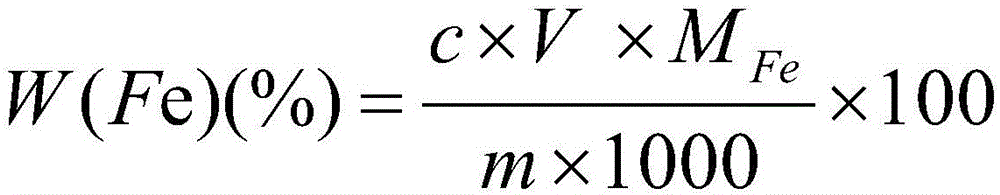Method for determination of iron in copper alloy
A determination method and technology for copper alloys, applied in the direction of chemical analysis by titration, can solve the problems of unsuitable measurement requirements for rapid, low-cost, high-efficiency, high use environment requirements, and lack of stability. Measurement procedure, easy observation, and high measurement efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
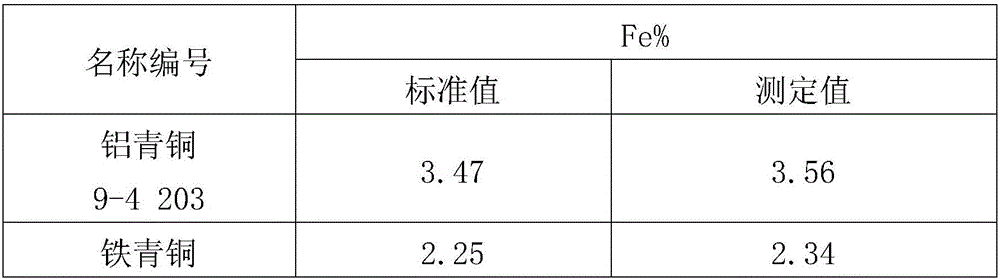
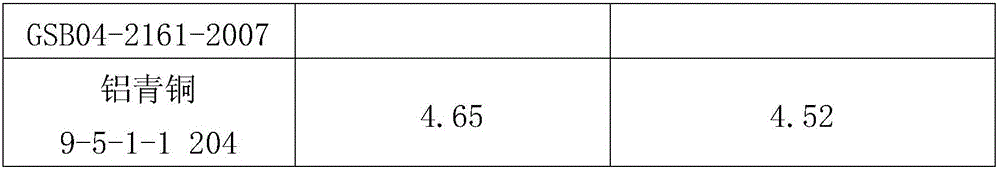
[0030] 1. Dissolution: Weigh 0.5000g copper alloy sample into a 400mL beaker, add 20mL concentrated HCl (density 1.19g / mL), 5mLH 2 o 2 (H 2 o 2 The mass fraction is 30%), heated and dissolved on a hot plate.
[0031] 2. Separation and precipitation: After the dissolution of the sample is completed, adjust the pH value to 7 with ammonia solution (the volume ratio of ammonia water with a density of 0.9g / mL to water is 1:1), and make an excess of 10mL, then filter through slow filter paper, and use ammonia solution (the volume ratio of ammonia water with a density of 0.9g / mL to water is 1:99) to wash until there is no copper ion, and the precipitate is washed into In a 300mL Erlenmeyer flask, wash the precipitate on the filter paper with hydrochloric acid (the volume ratio of concentrated hydrochloric acid with a density of 1.19g / mL to water is 1:99), and control the total volume to about 50mL.
[0032] 3. Redox titration: use tin dichloride (tin dichloride is dissolved in hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com