Recovery method of trifluoromethane
A technology of trifluoromethane and difluoromonochloromethane is applied in chemical instruments and methods, halogenated hydrocarbon preparation, organic chemistry, etc., and can solve problems such as environmental pollution, high production cost, ozone layer destruction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
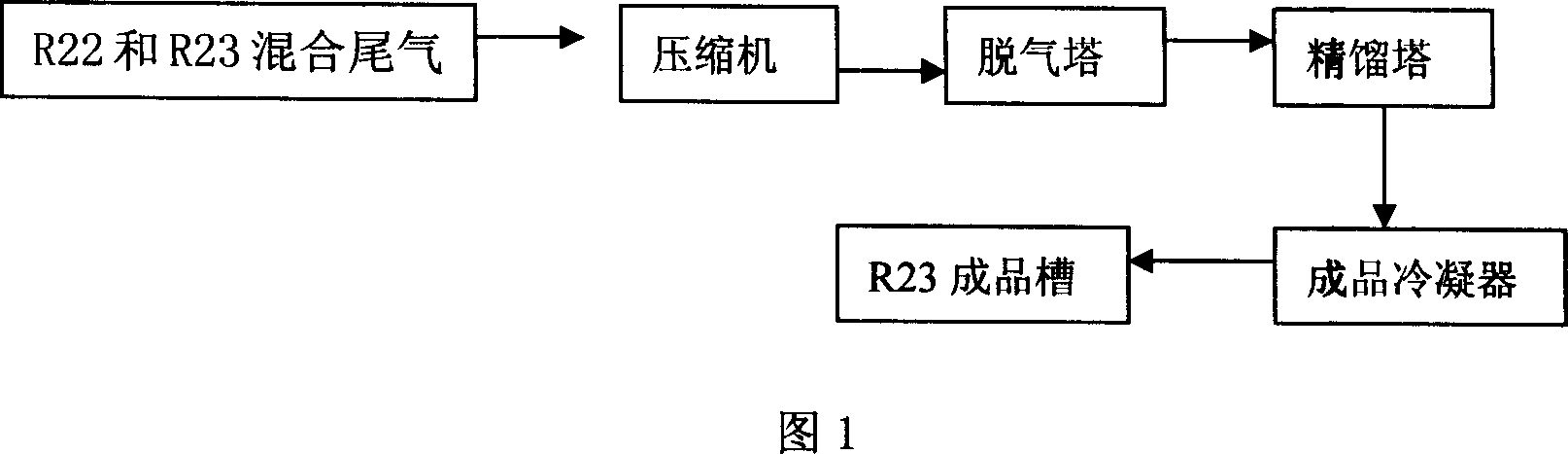
[0031] The exhaust gas (composed of difluorochloromethane: trifluoromethane = 65: 35 (weight ratio)) from the exhaust tower of the difluorochloromethane plant first enters the degassing tower after being compressed by the compressor, and the degassing tower The temperature is 25°C and the pressure is 3.5MPa. , to separate difluorochloromethane and trifluoromethane; then the separated crude trifluoromethane enters the rectification tower, the temperature of the rectification tower is 25° C., and the pressure is 3.5 MPa. Trifluoromethane with a purity of >99.5% is obtained. At the same time, difluorochloromethane with a purity of ≥95% was recovered. The trifluoromethane product from the top of the rectification tower is cooled and liquefied by the product condenser, and then collected in the trifluoromethane product tank. The condensing temperature of the finished condenser is 0°C, and the condensing pressure is 3.5MPa.
Embodiment 2
[0033] The exhaust gas (composed of difluorochloromethane: trifluoromethane = 65: 35 (weight ratio)) from the exhaust tower of the difluorochloromethane plant first enters the degassing tower after being compressed by the compressor, and the degassing tower The temperature is 30°C and the pressure is 4.5MPa. , to separate difluorochloromethane and trifluoromethane; then the separated crude trifluoromethane enters the rectification tower, the temperature of the rectification tower is 20°C, and the pressure is 3.5MPa. Trifluoromethane with a purity of >99.5% is obtained. At the same time, difluorochloromethane with a purity of ≥95% was recovered. The trifluoromethane product from the top of the rectification tower is cooled and liquefied by the product condenser, and then collected in the trifluoromethane product tank. The condensing temperature of the finished condenser is -10°C, and the condensing pressure is 4.5MPa.
Embodiment 3
[0035] The exhaust gas (composed of difluorochloromethane: trifluoromethane = 65: 35 (weight ratio)) from the exhaust tower of the difluorochloromethane plant first enters the degassing tower after being compressed by the compressor, and the degassing tower The temperature is 40°C and the pressure is 4.5MPa. , to separate difluorochloromethane and trifluoromethane; then the separated crude trifluoromethane enters the rectification tower, the temperature of the rectification tower is 20°C, and the pressure is 3.5MPa. Trifluoromethane with a purity of >99.5% is obtained. At the same time, difluorochloromethane with a purity of ≥95% was recovered. The trifluoromethane product from the top of the rectification tower is cooled and liquefied by the product condenser, and then collected in the trifluoromethane product tank. The condensing temperature of the finished condenser is -10°C, and the condensing pressure is 4.5MPa.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com