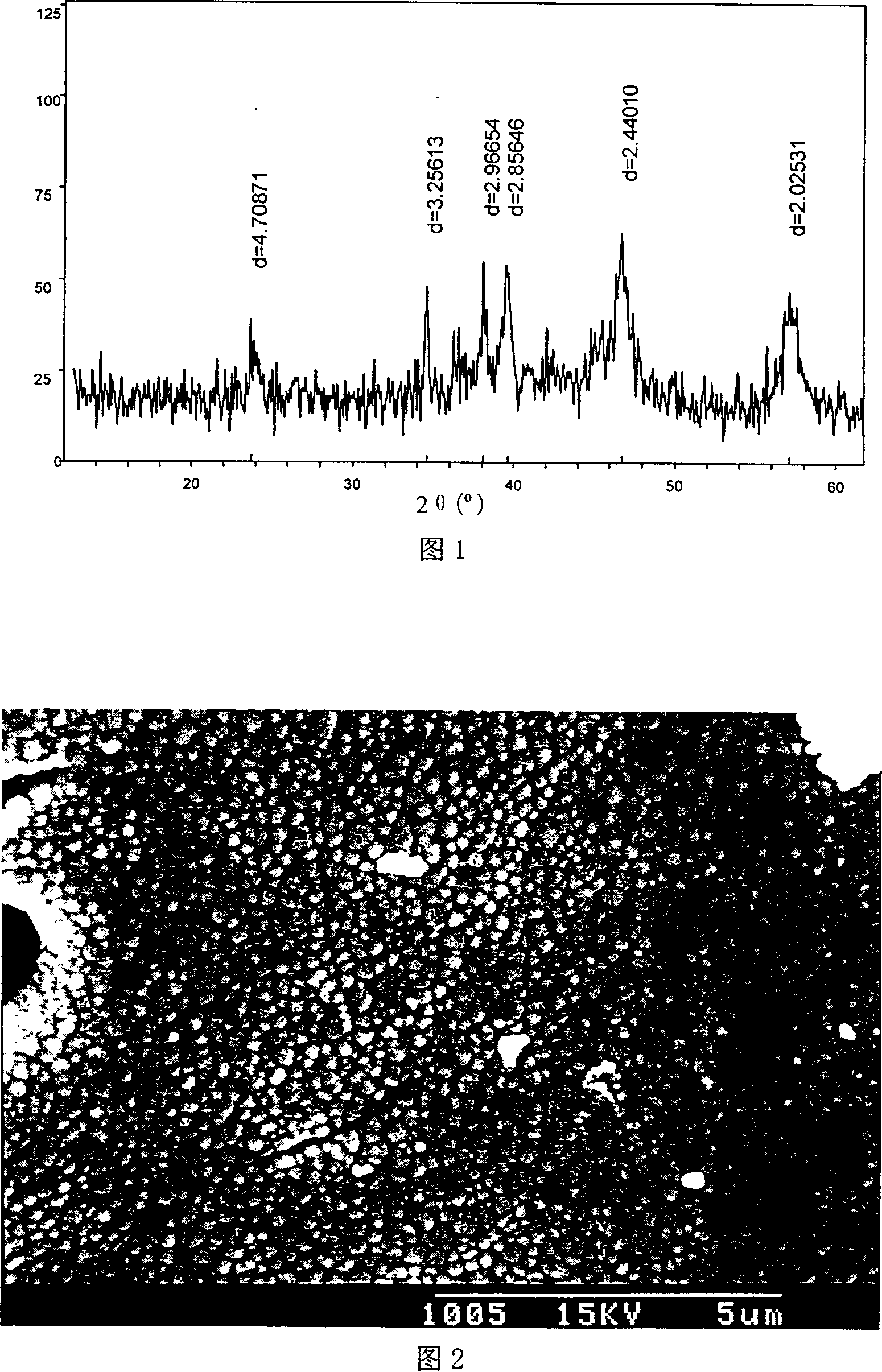
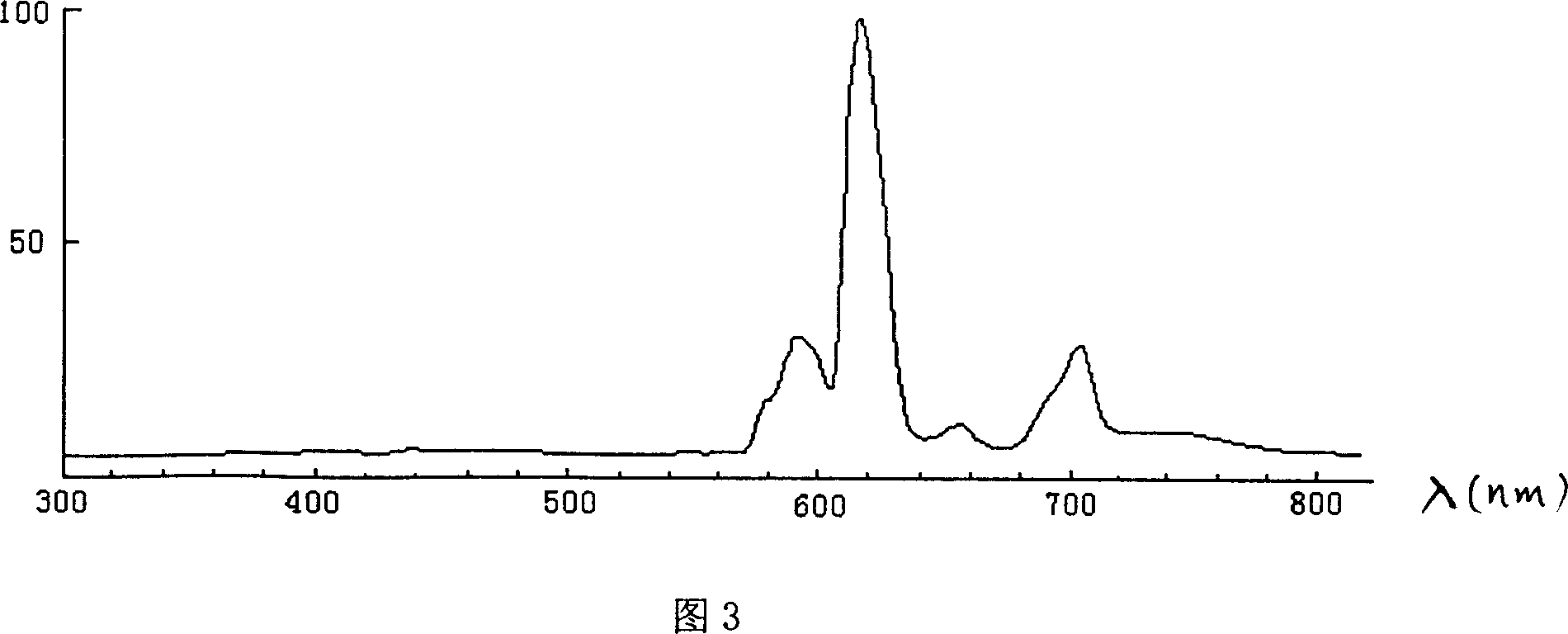
Aluminate red luminescent material and preparation process thereof
A red light-emitting, aluminate technology, applied in the direction of light-emitting materials, chemical instruments and methods, can solve the problems of complex preparation process, high product cost, application limitations, etc., to achieve good light-emitting performance, fast reaction speed and simple method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The chemical composition expression of aluminate red luminescent material is Mg 0.7 Ca 0.3 Al 2 o 4 :Eu 0.01 .
[0016] The preparation method is as follows:
[0017] 1) Take each nitrate by weight as follows:
[0018] Raw material Moles Weight (g) Molecular weight
[0019] Magnesium nitrate Mg(NO 3 ) 2 ·6H 2 O 0.7 178.1 254.41
[0020] Calcium nitrate Ca(NO 3 ) 2 4H 2 O 0.3 70.8 236.15
[0021] Aluminum nitrate Al(NO 3 ) 3 9H 2 O 2 750.3 375.13
[0022] Europium nitrate Eu(NO 3 ) 3 0.01 3.4 337.97
[0023] 2) Add 300 grams of urea to the raw material described in 1), and then add 650 grams of water, and fully stir at a temperature of 60° C. to obtain a precursor.
[0024] 3) Reacting the precursor obtained in 2) at a temperature of 500° C. for 30 minutes to obtain a reaction product.
[0025] 4) The reaction product obtained in 3) is taken out and cooled to room temperature, sieved with a 200-mesh sieve, and the sieve is taken to obtain th...
Embodiment 2
[0027] The chemical composition expression of aluminate red luminescent material is Mg 0.9 Ca 0.1 Al 2 o 4 :Eu 0.01 Dy 0.01 .
[0028] The preparation method is as follows:
[0029] 1) Take each nitrate by weight as follows:
[0030] Raw material Moles Weight (g) Molecular weight
[0031] Magnesium nitrate Mg(NO 3 ) 2 ·6H 2 O 0.9 229.0 254.41
[0032] Calcium nitrate Ca(NO 3 ) 2 4H 2 O 0.1 23.6 236.15
[0033] Aluminum nitrate Al(NO 3 ) 3 9H 2 O 2 750.3 375.13
[0034] Europium nitrate Eu(NO 3 ) 3 0.01 3.4 337.97
[0035] Dysprosium nitrate Dy(NO 3 ) 3 0.01 3.5 348.51
[0036] 2) Add 500 grams of urea to the raw material of 1), and then add 1500 grams of water, and stir thoroughly at a temperature of 50° C. to obtain a precursor.
[0037] 3) Reacting the precursor obtained in 2) at a temperature of 600° C. for 20 minutes to obtain a reaction product.
[0038] 4) The reaction product obtained in 3) is taken out and cooled to room te...
Embodiment 3
[0040] The chemical composition expression of aluminate red luminescent material is Mg 0.8 Ca 0.2 Al 2 o 4 :Eu 0.01 Ce 0.005 .
[0041] The preparation method is as follows:
[0042] 1) Take each nitrate by weight as follows:
[0043] Raw material Moles Weight (g) Molecular weight
[0044] Magnesium nitrate Mg(NO 3 ) 2 ·6H 2 O 0.8 203.5 254.41
[0045] Calcium nitrate Ca(NO 3 ) 2 4H 2O 0.2 47.2 236.15
[0046] Aluminum nitrate Al(NO 3 ) 3 9H 2 O 2 750.3 375.13
[0047] Europium nitrate Eu(NO 3 ) 3 0.01 3.4 337.97
[0048] Cerium nitrate Ce(NO 3 ) 2 ·6H 2 O 0.005 1.9 372.13
[0049] 2) Add 600 grams of urea to the raw material of 1), and then add 1500 grams of water, and stir thoroughly at a temperature of 70° C. to obtain a precursor.
[0050] 3) Reacting the precursor obtained in 2) at a temperature of 700° C. for 10 minutes to obtain a reaction product.
[0051] 4) The reaction product obtained in 3) is taken out and cooled to room temperatu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com