Chain-prolonged type fluorenyl bimaleimide and its preparation method
A bismaleimide and chain extension technology, which is applied in the field of chain extension fluorenyl bismaleimide and its preparation, can solve problems such as synthesis and solubility that have not been mentioned or discussed in detail
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
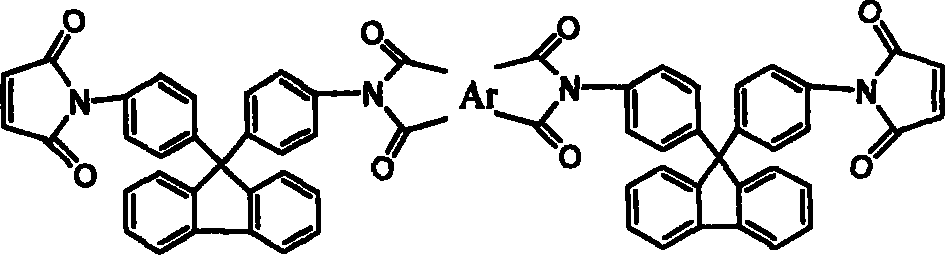
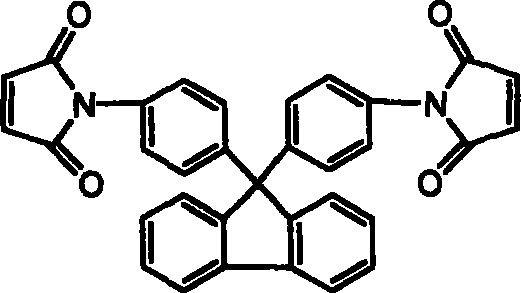
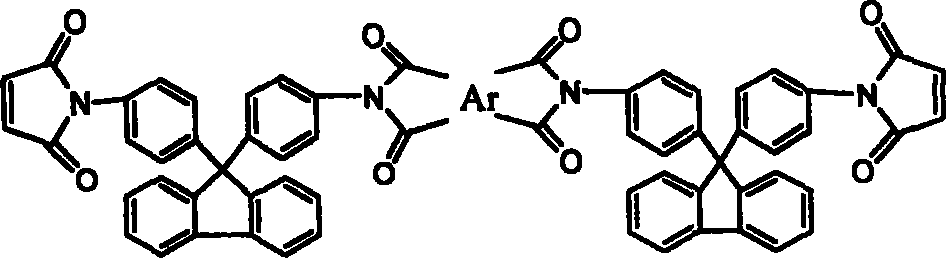
[0026] 17.4g (0.05mol) of 9,9-bis(4-aminophenyl)fluorene was dissolved in 120ml of N,N-dimethylacetamide (DMAc), cooled to 0°C in an ice bath, and equimolar The N,N-dimethylacetamide solution of maleic anhydride, after the dropwise addition was completed and reacted for 3 hours, directly added equimolar 3,3',4'4'-oxyethylene phthalic dianhydride (ODPA), After continuing to stir and react for 3 hours, add a mixture of acetic anhydride and potassium acetate, stir at room temperature for 1 hour, react at 60°C for 4 hours, and precipitate in water to obtain ether anhydride chain-extended fluorenyl bismaleimide.
Embodiment 2
[0028] Except for using 0.05mol 3, 3', 4'4'-benzophenone tetraacid dianhydride, other recipe operation steps and results are exactly the same as in Example 1, and the ketone anhydride chain extension type fluorenyl bismaleimide is obtained amine.
Embodiment 3
[0030] Except for using 0.05 mol of bisphenol A dianhydride, other formula operation steps and results are exactly the same as in Example 1, and bisphenol A dianhydride chain extension type fluorenyl bismaleimide is obtained.
[0031] The thermogravimetric analysis results of the fluorenyl bismaleimide obtained above are as shown in Table 3 and Figure 4 after thermal cyclization homopolymerization, and it can be seen that the cured resin heat of the chain extension type fluorenyl bismaleimide is The stability is better than that of the cured resin of the unextended monomer 9,9-bis(4-maleimidophenyl)fluorene. Therefore, the chain extension type fluorenyl bismaleimide can improve the processing performance while maintaining thermal stability, and the resin can be used as an additive component in various prepregs, composite materials and adhesives to improve the heat resistance of the material sex.
[0032] Table 3 Cured chain extension type fluorenyl bismaleimide thermogravimet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com