Fas associated factor 1
A technology related to factors and fragments, applied in the field of cell death, can solve problems such as unclear role
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0144] Example 1.2 - Cells and Reagents
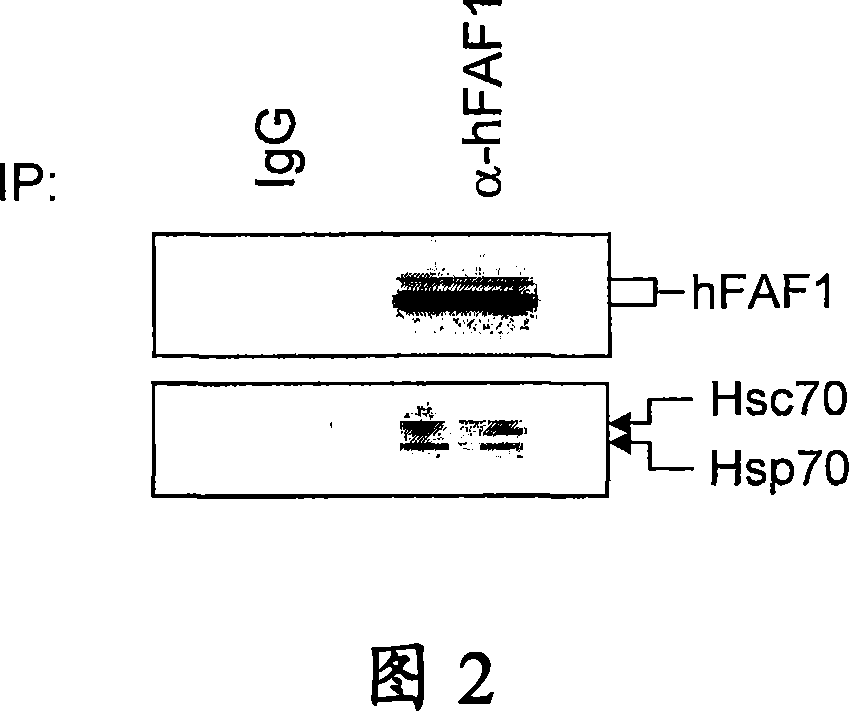
[0145] at 37°C and 5% CO 2 Human embryonic kidney epithelial (HEK293T) cells were grown and maintained in high-glucose Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum. Monoclonal anti-Flag antibody M2 was purchased from Sigma. Monoclonal anti-Hsc70 / Hsp70 antibody was obtained from StressGen. Polyclonal anti-hFAF1 antibody was prepared by our research group.
[0146] Example 1.3 - Transient transfection
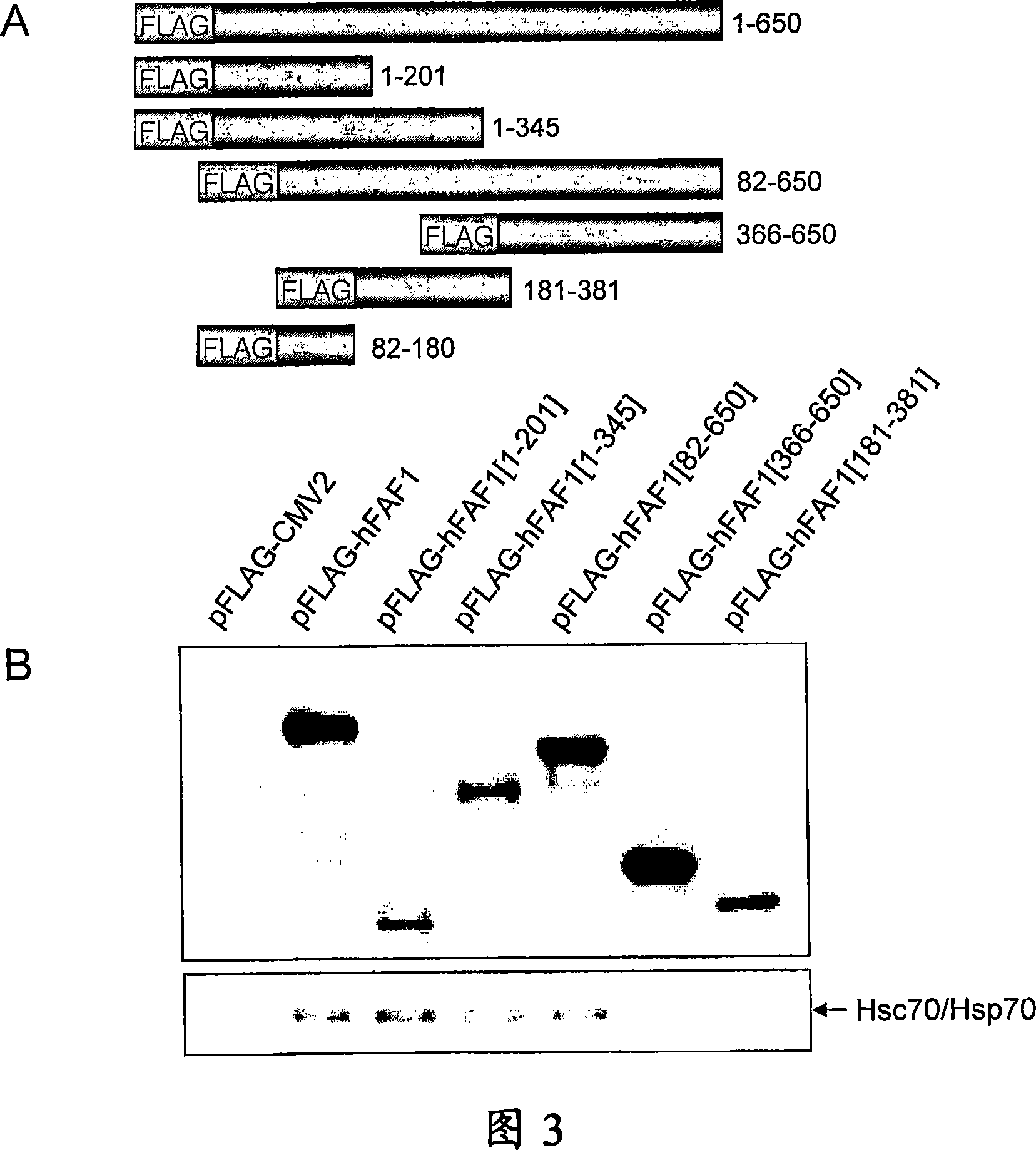
[0147] HEK293T cells were transfected with expression plasmids by calcium phosphate precipitation method. The day before transfection, cells were seeded on 10 cm dishes at a density of 1.5 × 10 6 cells were then transiently transfected with 6-12 μg of expression plasmid by means of calcium phosphate precipitation. The medium was replaced with fresh medium 6 hours after transfection, and cultured for an additional 18 hours.
Embodiment 14
[0148] Example 1.4 - Metabolic labeling and immunoprecipitation
[0149] Use 2 μCi / ml in methionine semi-free medium [ 35 S] Methionine metabolically labels cells for 18 hours. On ice with buffer containing protease inhibitors (50mM Tris, pH8.0, 150mM NaCl, 2mM EDTA, 0.5% NP-40, 1mM PMSF, 0.5mMDTT, 5μg / ml aprotinin, 1μg / ml leupeptin Peptide, 5mM Na 3 VO 4 , 5mMNaF) to disrupt the cells for 30 minutes. Centrifuge the lysate at 12,000 rpm for 1 h, then incubate the supernatant with monoclonal anti-Flag M2-affinity cross-linked agarose beads for 3 h at 4 °C, or incubate with anti-Flag antibody for 2 h and at 4 °C. Incubate with protein A beads for an additional 1 hour. The beads were washed three more times with 1 ml of lysis buffer.
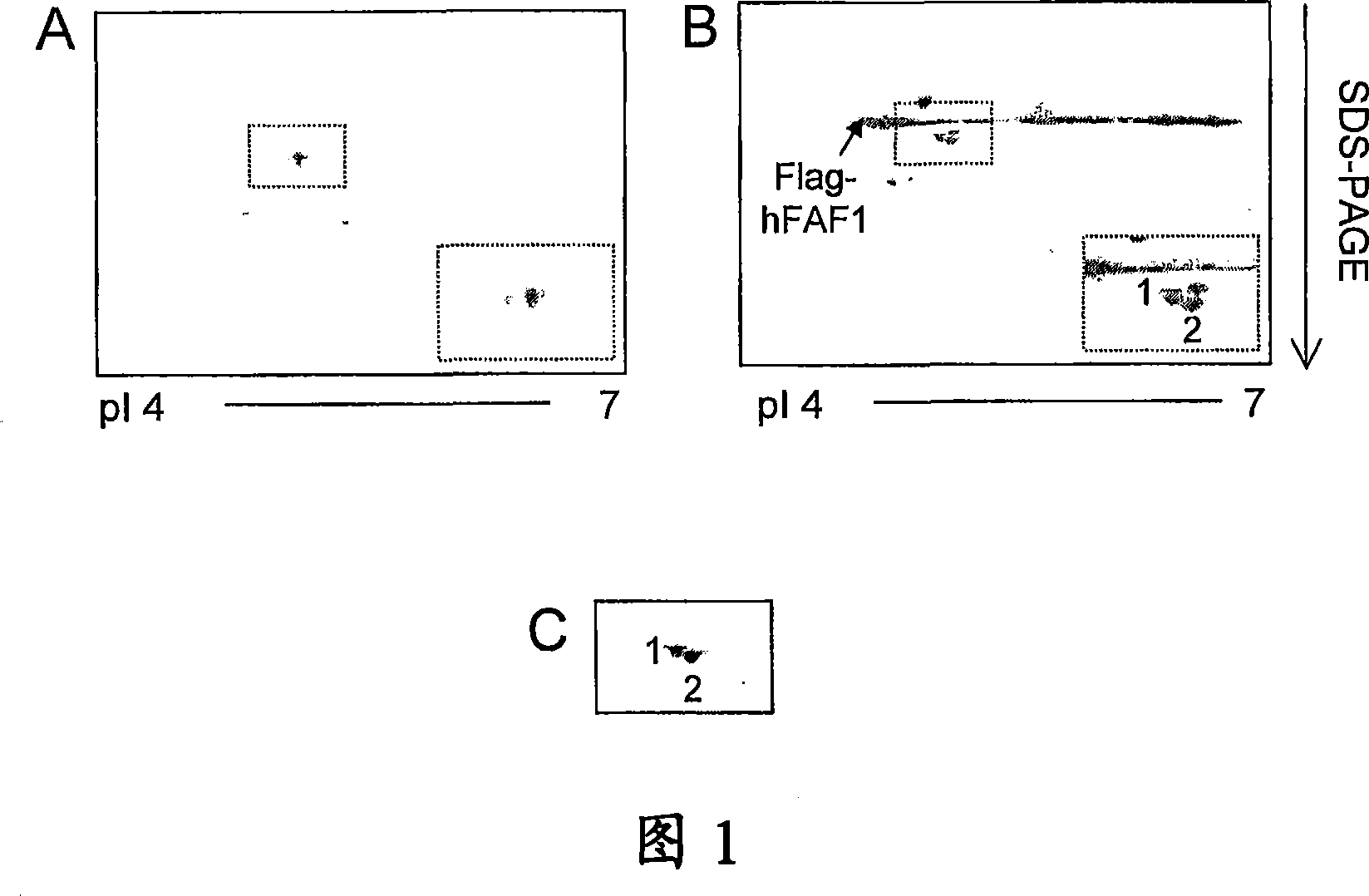
[0150] Example 1.5 - Two-dimensional gel electrophoresis
[0151] At room temperature with buffer (containing 9.5M urea, 2% Triton X-100, 5% β-mercaptoethanol, 1mM PMSF, 5μg / ml aprotinin, 10ug / ml pepstatin A, 10μg / ml light Aprotinin, 1 ...
Embodiment 17
[0154] Example 1.7 - Confocal Microscopy
[0155]For morphological studies, cells were grown on coated coverslips, transiently transfected, and then treated with heat shock. The cells were rinsed gently in HBSS and then fixed with 4% paraformaldehyde in HBSS for 10 minutes at room temperature. After washing with HBSS, cells were permeabilized by incubation with 0.1% Triton X-100 in HBSS for 10 min at room temperature before immunostaining. Nonspecific protein uptake was inhibited by incubating cells in HBSS (containing 3% BSA, 0.2% Tween 20, and 0.2% gelatin) for 1 hour. For Hsc70 / Hsp70 staining, cells were incubated with mouse monoclonal Hsc70 / Hsp70 antibody (StressGen) diluted 1:150 in HBSS (with 1% sucrose and 1% BSA) for 2 hours at 37°C. After washing the wash three times with HBSS, cells were incubated with Texas red-conjugated rabbit anti-mouse (Molecular Probes) diluted 1:200 for 1 hour, followed by washing with HBSS. Flag-hFAF1 was stained with FITC-labeled monocl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com