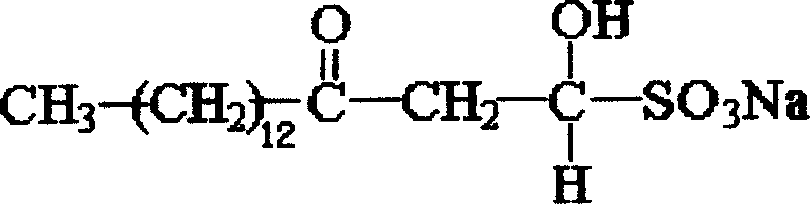Sodium myristyl aldehyde sulfate and its application as immunoadjuvant
A technology of sodium myristoyl acetaldehyde sulfite and immune adjuvant, which is applied in the direction of medical preparations containing active ingredients, anhydride/acid/halide active ingredients, antibody medical ingredients, etc., which can solve the problem of decline, poor antibody production, and inability Cryopreservation and other issues to achieve the effect of good safety, increased activity, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1, synthesize myristoyl acetaldehyde sodium sulfite according to the following steps:
[0020] ① Synthesis of intermediates
[0021] Add 1.5 kg of slaked lime, add 1.2 kg of water, and stir to form a paste; then add 0.6125 kg of myristic acid and 1.5 kg of acetic acid, mix well, pour into the lime paste, and keep stirring to form 2.5 kg of calcium salt. After dry distillation, 0.5 kg of crude product was obtained; the crude product was distilled under reduced pressure, and constant fractions were collected to obtain 0.377 kg of light yellow intermediate.
[0022] ②, grafting
[0023] 377 grams of intermediates and 20 grams of ethyl formate were dissolved in 2 liters of anhydrous benzene, and poured into a 5-liter round bottom flask. Then 5.76 g of sodium metal was chopped and added to the reaction liquid in portions, shaking from time to time, and keeping the temperature below 40°C. Leave overnight at room temperature. Extracted 4 times with 1500 ml of wa...
Embodiment 2
[0026] Embodiment 2, synthesize myristoyl acetaldehyde sodium sulfite according to the following steps:
[0027] ① Synthesis of intermediates
[0028] Add 1 kg of slaked lime, add 0.8 kg of water, and stir to form a paste; then add 0.5 kg of myristic acid and 1 kg of acetic acid, mix well, pour into the lime paste, and keep stirring to form 1.5 kg of calcium salt. After dry distillation, 0.3 kg of the crude product was obtained; the crude product was distilled under reduced pressure, and constant fractions were collected to obtain 0.25 kg of a light yellow intermediate.
[0029] ②, grafting
[0030] 250 grams of intermediate and 15 grams of ethyl formate were dissolved in 1.5 liters of anhydrous benzene, and poured into a 5-liter round bottom flask. Then chop up 4.6 grams of sodium metal, add to the reaction solution in portions, shake from time to time, and keep the temperature below 40°C. Leave overnight at room temperature. Extracted 3 times with 1000 ml of water, and c...
Embodiment 3
[0033] Embodiment 3, synthesize myristoyl acetaldehyde sodium sulfite according to the following steps:
[0034] ① Synthesis of intermediates
[0035] Add 2 kg of slaked lime, add 1.6 kg of water, and stir to form a paste; then add 0.75 kg of myristic acid and 2 kg of acetic acid, mix well, pour into the lime paste, and keep stirring to form 4 kg of calcium salt. After dry distillation, 0.9 kg of crude product was obtained; the crude product was distilled under reduced pressure, and constant fractions were collected to obtain 0.67 kg of light yellow intermediate.
[0036] ②, grafting
[0037] 670 grams of intermediates and 30 grams of ethyl formate were dissolved in 3 liters of anhydrous benzene, and poured into a 5-liter round bottom flask. Then 7.6 g of sodium metal was chopped and added to the reaction liquid in portions, shaking from time to time, and keeping the temperature below 40°C. Leave overnight at room temperature. Extracted 3 times with 1800 ml of water, and c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com