High spermidine conjugates, preparation and application thereof
A technology of homospermidine conjugates, which is applied in the field of preparation of homospermidine conjugates, can solve the problems of toxic side effects, lack of selectivity of drug molecules to diseased cells, etc., and achieve low price, high yield, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
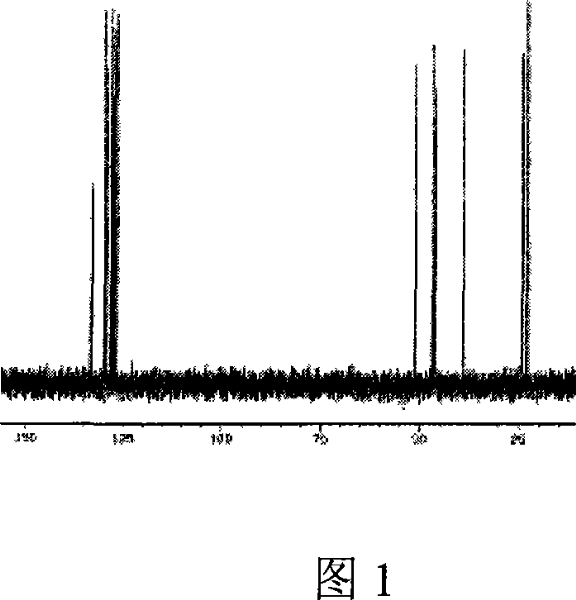
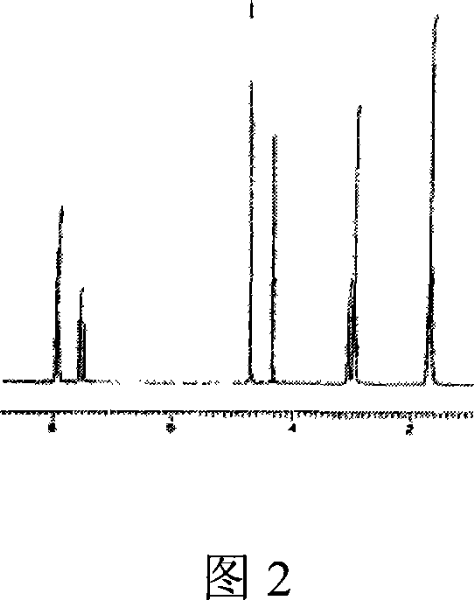
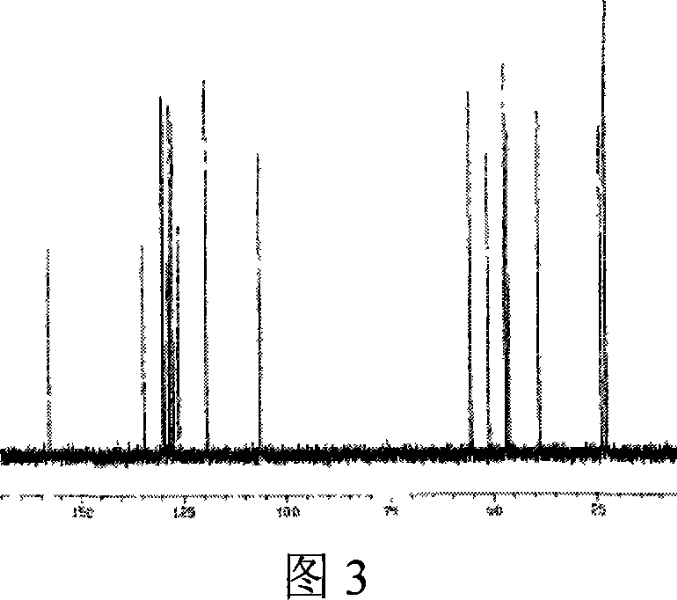
Image
Examples
Embodiment 1
[0054] Example 1 Preparation of a homospermidine intermediate whose one end and the middle amino group are protected by BOC
[0055] Dissolve 8.99g of N-tert-butoxycarbonyl-1,4-butanediamine in 180mL of acetonitrile, add 9g of anhydrous potassium carbonate, stir at room temperature for 15min, raise the temperature to 45°C, and add N-(4-bromobutyl A total of 11.5 g of phthalimide was reacted overnight at a temperature of 45°C. Acetonitrile was distilled off under reduced pressure, the residue was extracted with 30 mL chloroform, and 10% Na 2 CO 3 The aqueous solution was washed three times with 40 mL each time, the organic layer was collected, dried over anhydrous sodium sulfate, and the chloroform was distilled off under reduced pressure to obtain a pale yellow oil (containing impurities). Dissolve the oil in 100 mL of methanol solution, add 10.42 g of BOC anhydride, and stir overnight at room temperature until a large amount of white insoluble solids are formed. The solven...
Embodiment 2
[0058] Example 2 Preparation of Ar represented by general formula 1 is β-naphthyl, R 1 for NH 2 .3 Homospermidine conjugates at HCl:
[0059] According to the method for embodiment 1, Ar is prepared as β-naphthyl, R 1 When it is the homospermidine compound (intermediate) when it is H, take 0.09g of the above-mentioned homospermidine intermediate and dissolve it in 15mL of 25% methanol in dichloromethane solution, and add 0.14g of β-naphthaldehyde under stirring; -10°C Stir the reaction until the TLC detects that the raw material point of β-naphthaldehyde is weakened, then stop the reaction, evaporate the solvent under reduced pressure, dissolve the residue in 12 mL of 50% methanol in dichloromethane, and stir the reaction mixture at -10°C 15min, add NaBH three times 4 A total of 0.1g was reacted at -10°C for 4h, the solvent was evaporated under reduced pressure, the residue was dissolved in 20mL chloroform, and 10% Na 2 CO 3 The aqueous solution was washed three times wit...
Embodiment 3
[0062] Example 3 Preparation of Ar shown in Formula 1 is 6-methoxynaphthyl, R 1 for NH 2 .3 Homospermidine conjugates at HCl:
[0063] According to the method of embodiment 1, Ar is 6-methoxynaphthyl, R 1 When the homospermidine intermediate is H, dissolve 0.3g of the above-mentioned homospermidine intermediate in 15mL of 25% methanol in dichloromethane solution, add 0.02g of 6-methoxynaphthaldehyde under stirring; stir at 60°C After 18 hours, the reaction was stopped when the raw material point of 6-methoxynaphthaldehyde disappeared as detected by TLC. The solvent was evaporated under reduced pressure, and the residue was dissolved in 12 mL of 50% methanol in dichloromethane solution, and the reaction mixture was stirred at 20°C for 15 min, and NaBH was added three times. 4 A total of 0.09g was reacted at 60°C for 18h, the solvent was evaporated under reduced pressure, the residue was dissolved in 20mL chloroform, and 10% Na 2 CO 3 The aqueous solution was washed three t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com