Extracorporeal induction process for differentiating hemopoietic stem/ancestral cell into mature red blood cell and its application
A mature red blood cell and hematopoietic stem technology, applied in the field of biomedicine, can solve problems such as safety doubts and low red blood cell denuclearization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0054] Example 2 Isolation of CD34+ cells from MNC (using miniMACS separation system)
[0055] 2.1 per 10 8Each umbilical cord blood mononuclear cell (MNC) was suspended in 300 μL 4°C preset PBS, 100 μL non-specific blocking antibody FcR blocking agent was added, and mixed. Then add 100 μL magnetic bead-coupled CD34 monoclonal antibody (FcR blocking agent and magnetic bead-coupled CD34 monoclonal antibody were purchased from Miltenyi Biotec, Lot #5050601032), mix well, and incubate at 4°C for 30 min;
[0056] 2.2 Add 500μl PBS, centrifuge at 1,500rpm for 3min at 4C, discard the supernatant;
[0057] 2.3 Resuspend the cells with 1 mL of degassed PBS to prepare a single cell suspension;
[0058] 2.4 Fix the MACS separation column in the MACS magnetic field (both the MACS separation column and the magnetic field were purchased from MiltenyiBiotec), and rinse the separation column with 2 mL of degassed PBS;
[0059] 2.5 Slowly add the single cell suspension to the separation co...
Embodiment 3
[0061] Example 3 Analysis of the purity of hematopoietic stem cells
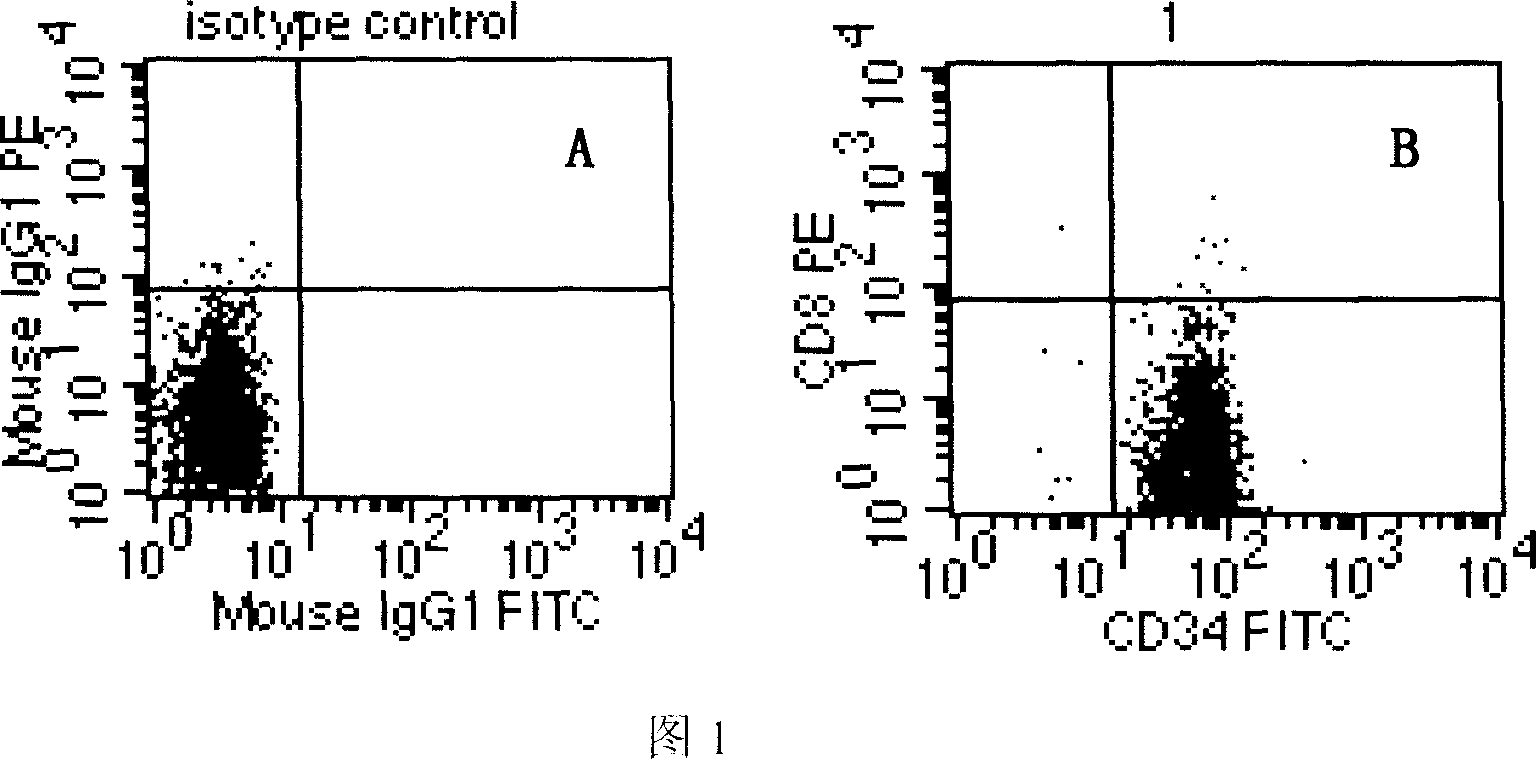
[0062] The immunomagnetic bead-labeled cells were purified twice through the separation column, and divided into two groups, one group was the test group, and the isolated CD34 was detected with PE-labeled anti-CD34 antibody (Miltenyi Biotec company product). + Cell purity, the other group is the control group, using PE-labeled anti-mouse IgG (product of Zhongshan Company) and isolated CD34 + Cell incubation was used as a control group, and CD34 was detected by flow cytometry + The purity of the cells can reach 98.64% (Figure 1).
Embodiment 4
[0063] Example 4 Isolation and culture of stromal cells derived from fetal liver
[0064] With the consent of the abortion patient himself, take the aborted fetus at 14 weeks of gestational age, separate the liver tissue, perfuse the blood vessel with normal saline, fully flush out the blood in the tissue, take a tissue block of appropriate size, remove the fascia, etc., and use sterilized scissors Mince the liver tissue into 1mm 3 size, arrange it in 25mm 2 20 pieces of tissue were planted in each flask, and then placed upside down in 5% CO 2 In the incubator, turn the culture bottle over after half an hour, add about 3mL medium, put it in the incubator to continue the culture, after about 2 days, cells can be seen crawling out from the edge of the tissue block, continue to culture when the cells cover 80% of the bottom of the culture bottle , digest it with 0.25% trypsin (Gibco company product, Cat#25200-056), plant it into a new bottle together with the tissue pieces, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com