Preparing blood vessel graft containing active components
An active ingredient, vascular transplantation technology, applied in anticoagulant treatment, packaging item types, special packaging items, etc., can solve the problems of sepsis, organ loss, patient death, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
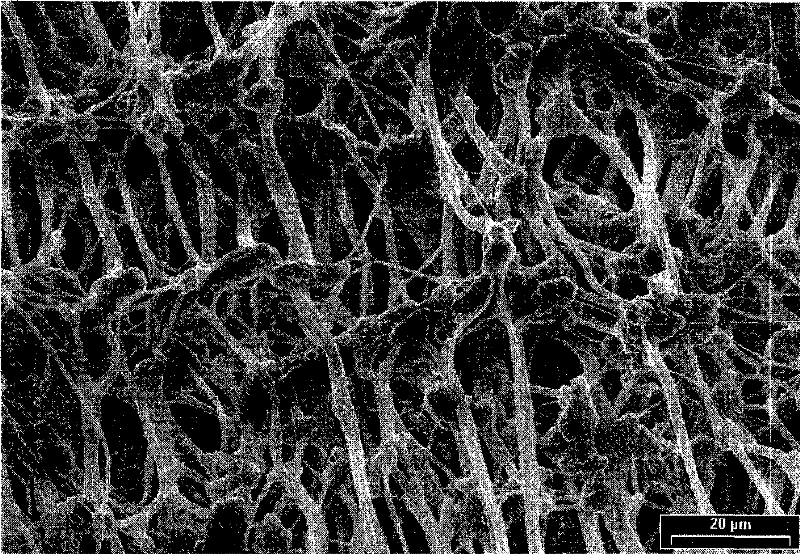
[0024] Vascular prostheses made of expanded PTFE (6 mm in diameter) were dipped in a 5% by weight solution of gentamicin palmitate in methanol for 60 seconds at room temperature. Then, the vascular PTFE prosthesis was dried at room temperature until a constant mass was achieved. The applied gentamicin palmitate coating was measured gravimetrically. The results showed a loading of 0.95 mg gentamicin palmitate per cm of vascular PTFE prosthesis. Scanning electron micrographs of coated vascular PTFE prostheses are shown in figure 1 middle.
Embodiment 2
[0026] Vascular prostheses made of expanded PTFE (diameter 6 mm) were dipped in 5% by weight gentamicin palmitate in methanol (containing 1% by weight argatro plaque) for 60 seconds at room temperature. Then, the vascular PTFE prosthesis was dried at room temperature until a constant mass was achieved. The applied gentamicin palmitate coating was measured gravimetrically. The results showed a gentamicin palmitate loading of 0.97 mg per cm of vascular PTFE prosthesis.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com