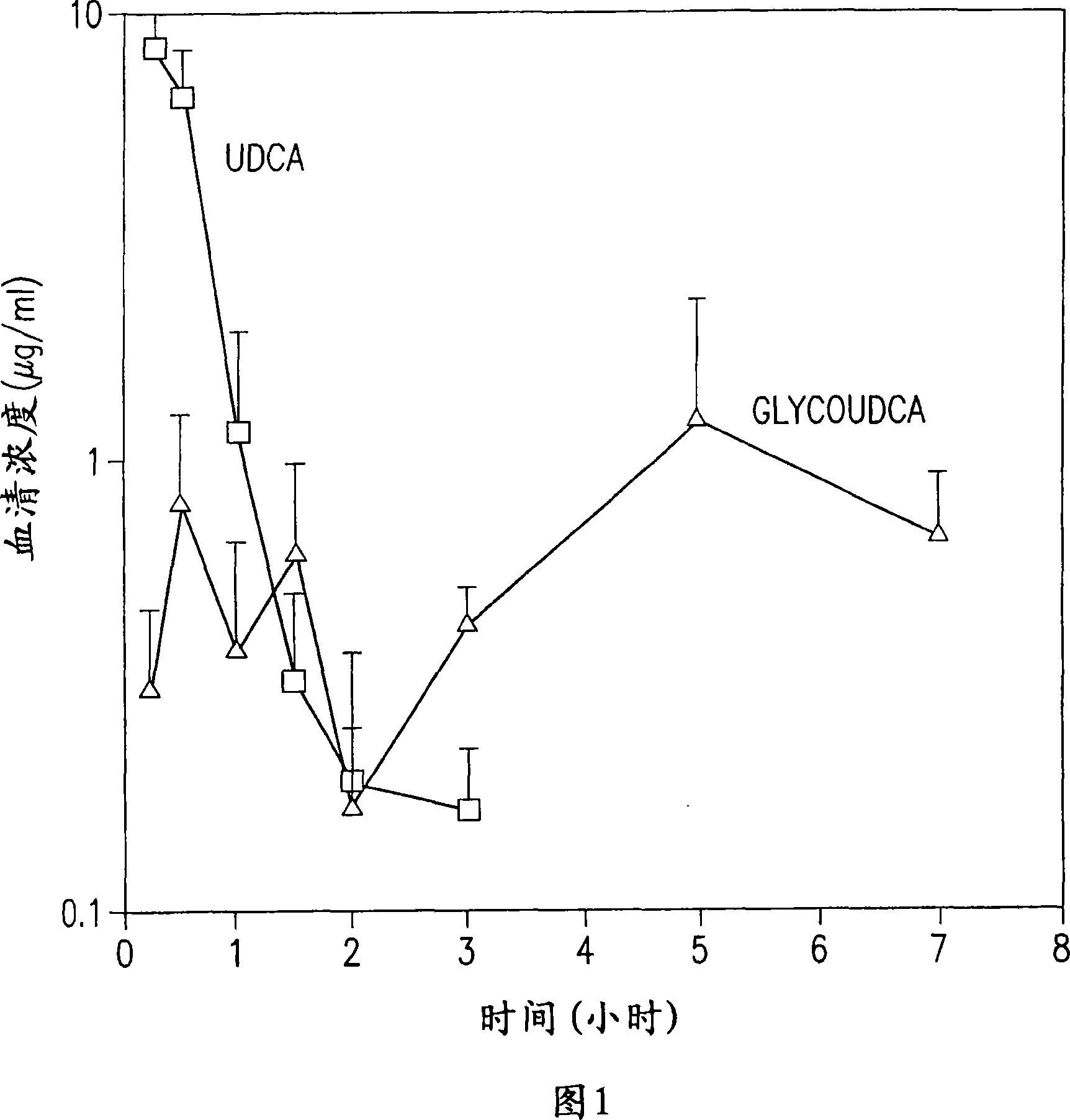
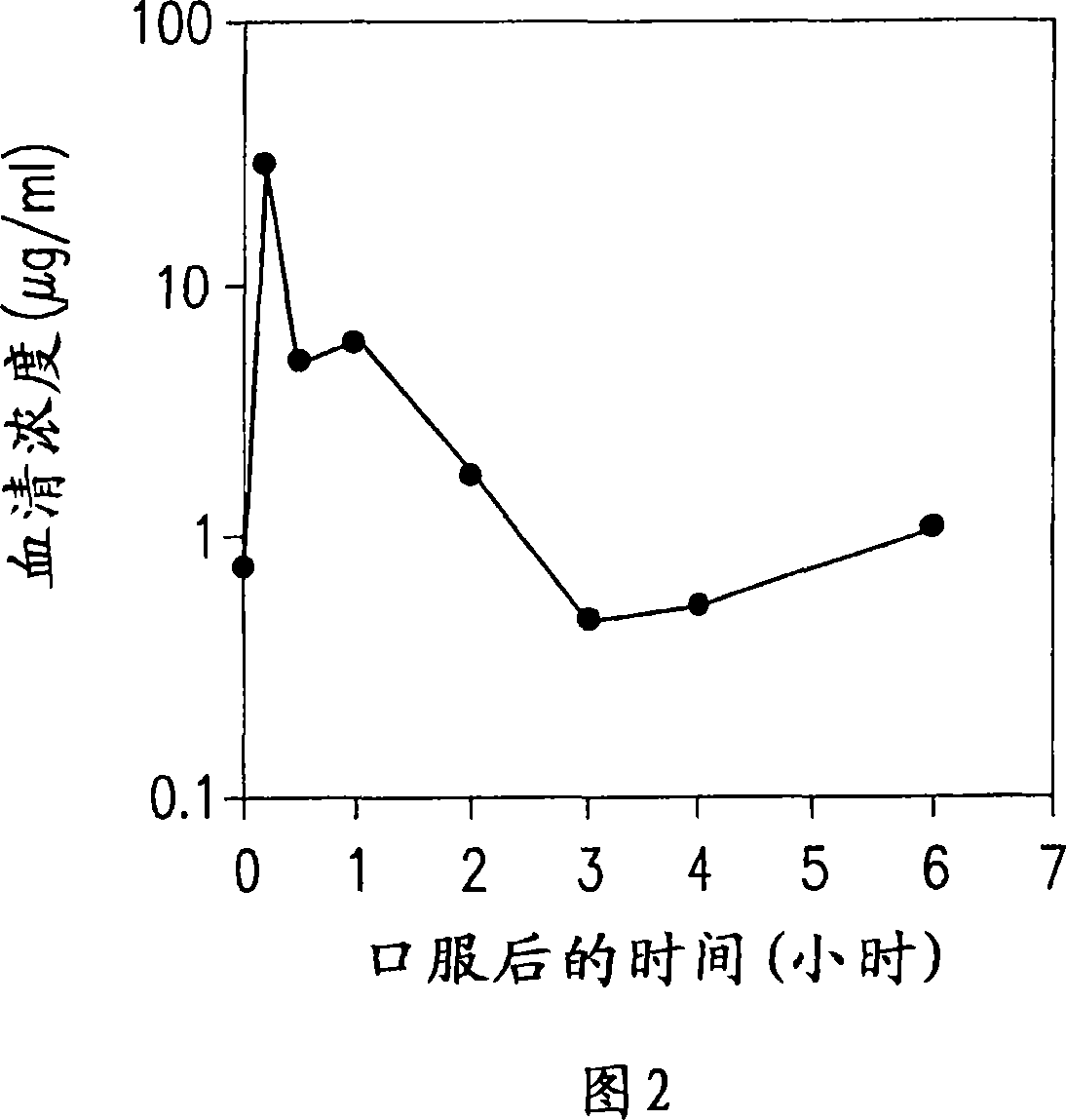
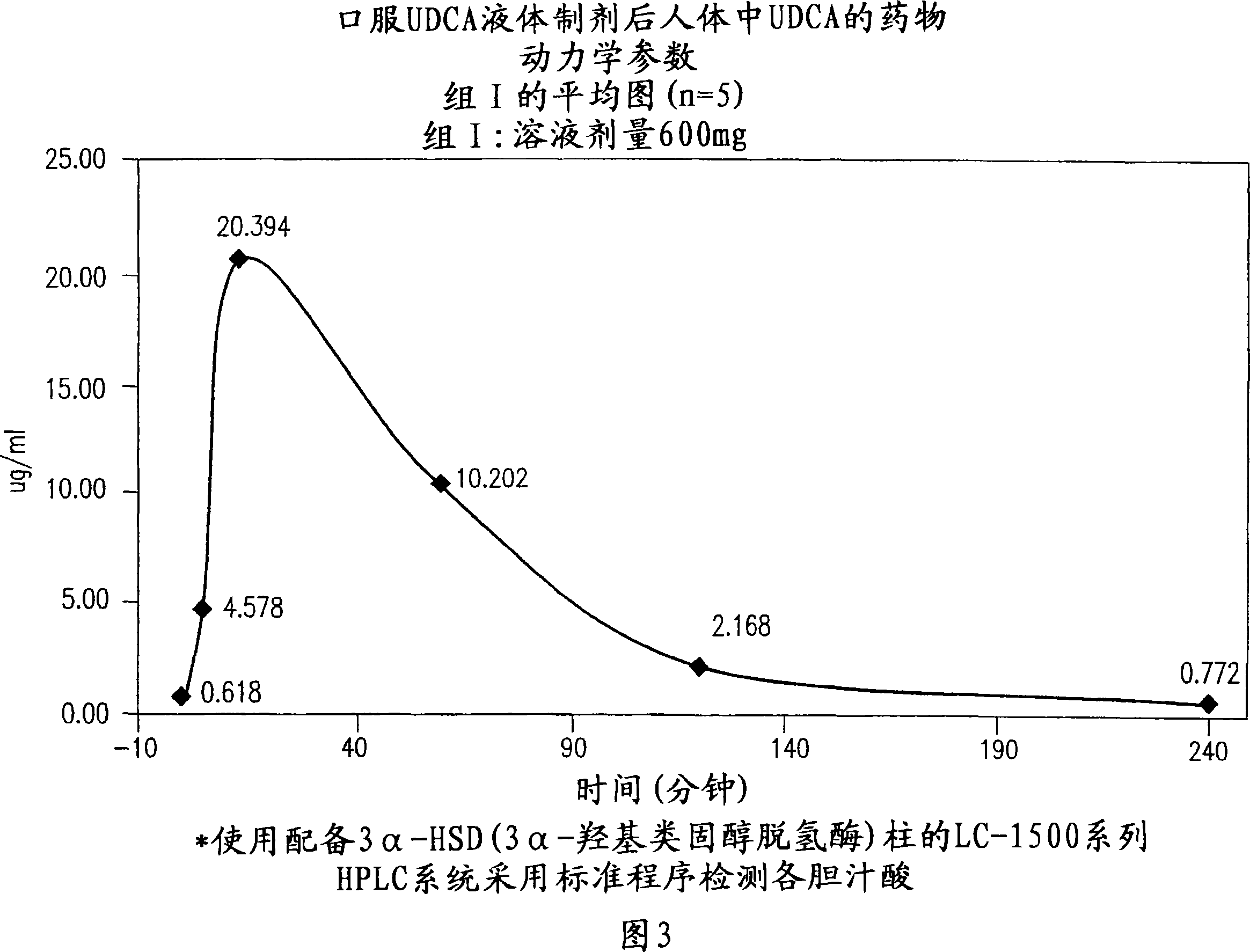
Dried forms of aqueous solubilized bile acid dosage formulation, preparation and uses thereof
A technology of bile acids and bile salts, which is applied in the direction of bulk delivery, antibacterial drugs, digestive system, etc., and can solve problems such as insufficient bioavailability, unstable water-based dosage forms, and limited uses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0078] The first series of solution formulations prepared according to the instructions below using soluble bile acids (as free acids) and high molecular weight water-soluble starch conversion products did not exhibit any precipitation at any of the pHs tested.
[0079] Soluble bile acids Starch conversion products (min)
[0080] If it is 200mg CDCA about 30g
[0081] If it is 200mg UDCA about 5g
[0082] If it is 200mg KLCA about 12g
[0083] If it is 200mg cholic acid, about 10g
[0084] If it is 200mg deoxycholic acid, about 50g
[0085] If it is 200mg hyodeoxycholic acid about 3.5g
[0086] Add pure water to 100mL
[0087] An aqueous solution (100 mL) in which one of the above-mentioned soluble bile acids was dissolved was prepared. Maltodextrin (as a high molecular weight conversion product of water-soluble starch) is dissolved with stirring at about 60-80°C to obtain a clear solution. The pH of the resulting clear solution is adjusted with acid to prepare oral dos...
Embodiment II
[0090] A second series of solution formulations prepared according to the instructions below using soluble bile acids (as free acids) and high molecular weight water-soluble starch conversion products did not exhibit any precipitation at any of the pHs tested.
[0091] Soluble bile acids Starch conversion products (min)
[0092] If it is 200mg CDCA about 18g
[0093] If it is 200mg UDCA about 3g
[0094] If it is 200mg KLCA about 7.2g
[0095] If it is 200mg cholic acid, about 6g
[0096] If it is 200mg deoxycholic acid, about 30g
[0097] If it is 200mg hyodeoxycholic acid about 2.1g
[0098] Add pure water to 100mL
[0099] An aqueous solution (100 mL) in which one of the above-mentioned soluble bile acids was dissolved was prepared. To the resulting solution was added maltodextrin (as a high molecular weight water-soluble starch conversion product) and dissolved with stirring at room temperature to give a clear solution. Oral dosage forms, topical formulations and so...
Embodiment III
[0102] The first series of solution formulations prepared according to the instructions below using soluble bile acids (as free acids) and high molecular weight water-soluble starch conversion products did not show any precipitation at pH 6.5-8.
[0103] Soluble bile acids Starch conversion products (min)
[0104] If it is 200mg CDCA about 15g
[0105] If it is 200mg UDCA about 1.5g
[0106] If it is 200mg KLCA about 3.6g
[0107] If it is 200mg bile acid, about 3g
[0108] If it is 200mg deoxycholic acid, about 15g
[0109] If it is 200mg hyodeoxycholic acid about 3.5g
[0110] Add pure water to 100mL
[0111] An aqueous solution (100 mL) in which one of the above-mentioned soluble bile acids was dissolved was prepared. To the resulting solution was added maltodextrin (as a high molecular weight water-soluble starch conversion product) and dissolved with stirring at room temperature to give a clear solution. Injectable, colon-localized, topical, and eye drop dosage for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com