Positive electrode active material for nonaqueous electrolyte secondary battery
一种正极活性材料、非水电解质的技术,应用在非水电解质蓄电池、二次电池、电池电极等方向,能够解决操作电压低等问题,达到操作电压高、性能优异的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] (1) Synthesis of positive electrode active materials
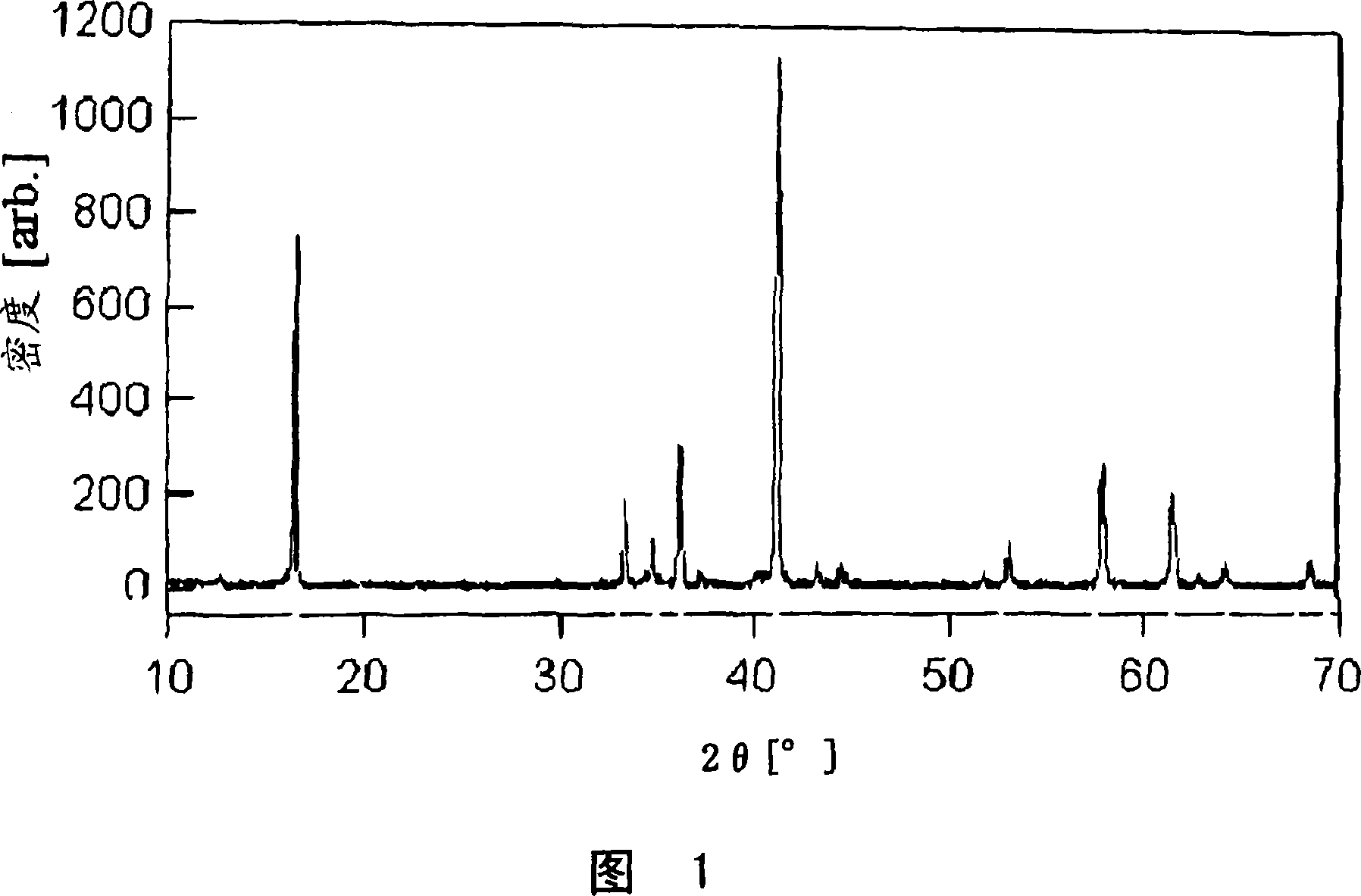
[0073] Weigh Na 2 CO 3 , Ni(OH) 2 and TiO 2 so that Na, Ni and Ti have NaNi 0.5 Ti 0.5 o 2 stoichiometric ratios and subsequently mix them thoroughly by means of an agate mortar. The resulting mixture was kept at 700° C. for 12 hours in an argon atmosphere to perform preliminary firing, and thereafter the mixture was kept at 950° C. for 36 hours to be fired to obtain a positive electrode for a nonaqueous electrolyte secondary battery Active substance A1. Al was subjected to powder X-ray diffraction, and the measured results are shown in FIG. 1 . Al has a hexagonal crystal structure and the value obtained by dividing the XRD peak intensity of 2.20 Ȧ interplanar spacing by the XRD peak intensity of 5.36 Ȧ interplanar spacing is 1.5. The XRD peak intensity of 2.09 Ȧ interplanar spacing divided by the XRD of 2.20 Ȧ interplanar spacing A value of 0.03 was obtained for the peak intensity.
[0074] (2) Evaluation...
Embodiment 2
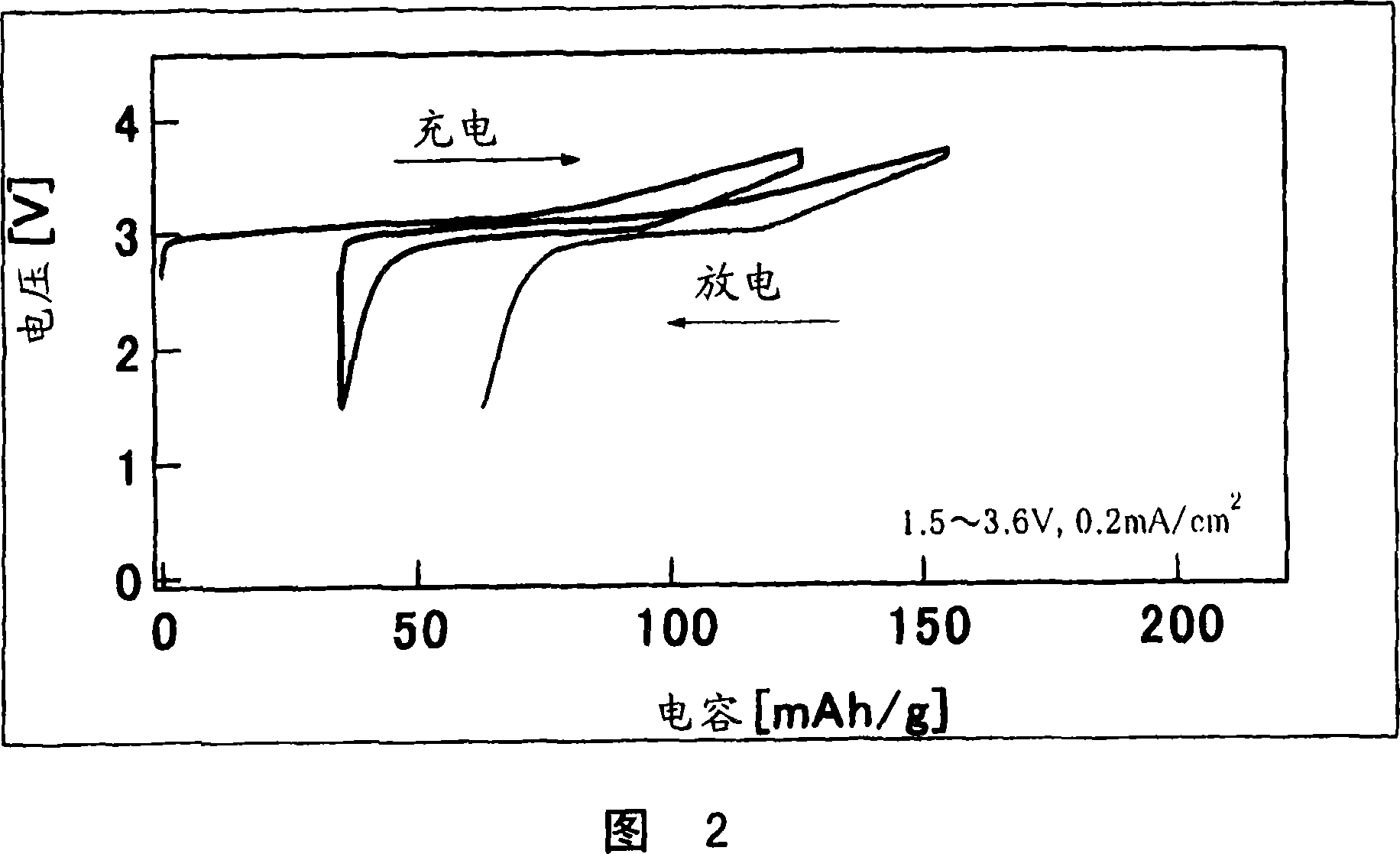
[0080] Using the Al obtained in Example 1, the same test cell as in Example 1 was fabricated and subjected to a constant current charge and discharge test under the following conditions.
[0081] Current density: 0.2mA / cm 2
[0082] Scan potential range: 1.5V-4.0V
[0083] The charge and discharge curves obtained at the first cycle and the second cycle are shown in FIG. 3 .
Embodiment 3
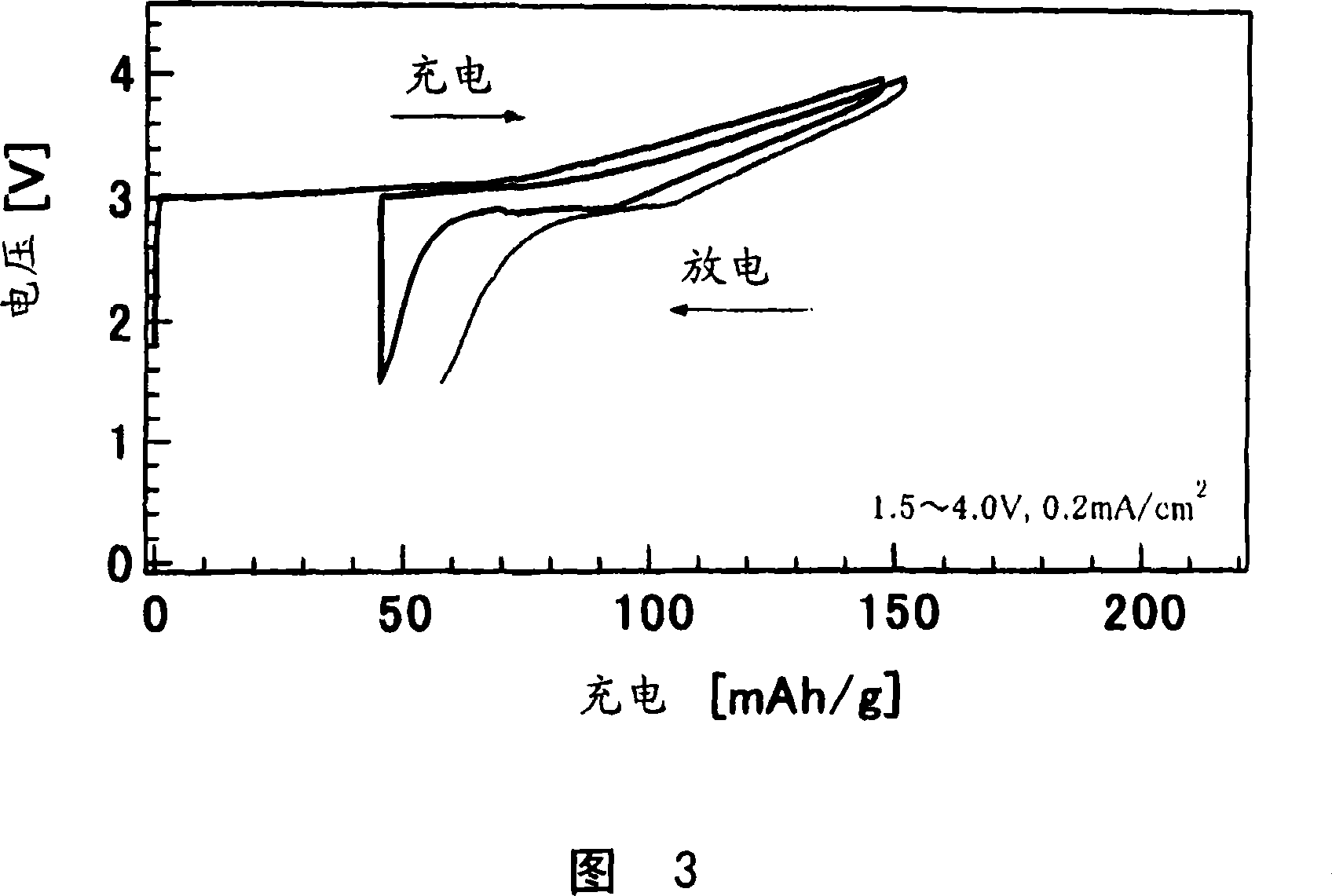
[0085] Using the Al obtained in Example 1, the same test cell as in Example 1 was fabricated and subjected to a constant current charge and discharge test under the following conditions.
[0086] Current density: 0.2mA / cm 2
[0087] Scan potential range: 1.5V-4.2V
[0088] The charge and discharge curves obtained at the first cycle and the second cycle are shown in FIG. 4 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com