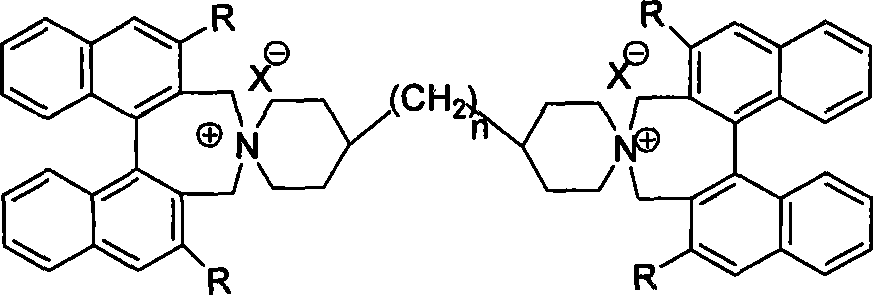
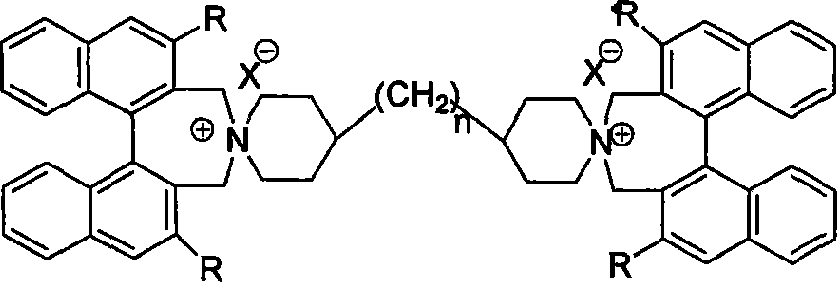
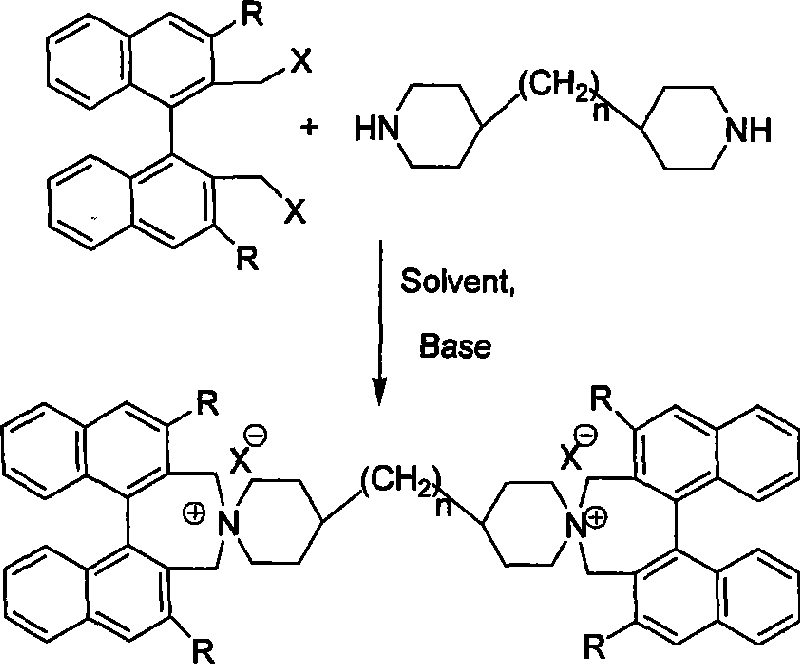
Phase-transferring catalyst containing dinaphthalene chiral double-spiral quaternary ammonium and its production
A technology of phase transfer catalysts and double spiro rings, which can be used in organic chemistry methods, chemical instruments and methods, physical/chemical process catalysts, etc., and can solve problems such as inability to obtain ideal results from substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0016] Example 1: Preparation of catalyst: (R, R)-bis(1,1'-binaphthyl-2,2'-methyleneamine)-4,4'-bipiperazine alkyl ammonium bromide
[0017]
[0018] (R, R)-1,1'-binaphthyl-2,2'-dimethyl bromide 660mg, (1.4mmol), 4,4'-bipiperazine 169mg (0.7mmol), potassium carbonate 580mg, ( 4.2mmol) into a 100mL round-bottomed flask, and 15mL of acetonitrile was added, the mouth of the round-bottomed flask was connected to a spherical condenser with a drying tube, heated to reflux at 80°C for 24 hours, and the reaction was detected by thin-layer chromatography. The reaction solution was poured into a beaker filled with 40 mL of water, and stirred for 10 minutes. Transfer to a separatory funnel and extract three times with 120 mL of dichloromethane. The organic phases were combined and dried over anhydrous magnesium sulfate. Filtration, evaporation of the solvent, quick silica gel column separation (dichloromethane / methanol=50 / 1 as the eluent), to obtain the target (R, R)-bis(1,1'-binaph...
example 2
[0020] Example 2: Preparation of catalyst: (S, S)-bis(1,1'-binaphthyl-2,2'-methyleneamine)-4,4'-bipiperazine ammonium bromide
[0021]
[0022] In a method similar to Example 1, using (S, S)-1,1-binaphthalene-2,2'-dimethyl bromide as a raw material, using tetrahydrofuran as a solvent, and a reaction temperature of 0°C, synthesize (S, S) -Bis(1,1'-binaphthyl-2,2'-methyleneamine)-4,4'-bipiperazine ammonium bromide, yield 91.2%. 1 H NMR (500MHz, CDCl 3 ), δppm: 1.72-1.77 (m, 2XCH, 2H), 1.81-1.88 (m, 2XCH, 2H), 2.10-2.22 (m, 2XCH, 2H), 2.29-2.33 (m, 2XCH, 2H), 2.35- 2.37(m, 2XCH, 2H), 3.37-3.42(m, 2XCH, 2H), 3.52-3.55(m, 2XCH, 2H), 3.63-3.74(m, 6XCH, 6H), 3.96(d, J=13.0Hz , 2H), 4.50(d, J=13.0Hz, 2H), 5.09(d, J=13.0Hz, 2H), 7.38-7.48(m, Ar-H, 8H), 7.62-7.65(m, Ar-H , 4H), 7.83-7.91 (m, Ar-H, 4H), 8.12-8.13 (m, Ar-H, 4H), 8.22-8.25 (m, Ar-H, 4H).
[0023] The catalyst is used to catalyze the conjugated addition reaction of nitroisopropane to α, β-unsaturated carbonyl compoun...
example 3
[0024] Example 3: Preparation of catalyst: (R, R)-bis(1,1'-binaphthyl-2,2'-methyleneamine)-4,4'-bispiperazine methane ammonium bromide
[0025]
[0026] In a similar manner to Example 1, using 4,4'-bispiperazine methane as a starting material, reacting at 25 ° C, using 1.4 mmol sodium carbonate as a base, and using chloroform as a solvent, synthesize (R, R)-bis(1 , 1'-binaphthyl-2,2'-methyleneamine)-4,4'-bispiperazine methane ammonium bromide, the yield was 88.3%. 1 H NMR (500MHz, CDCl 3 ), δppm: 1.19-1.22 (m, CH 2, 2H), 1.72-1.77(m, 2XCH, 2H), 1.81-1.88(m, 2XCH, 2H), 2.10-2.22(m, 2XCH, 2H), 2.29-2.33(m, 2XCH, 2H), 2.35- 2.37(m, 2XCH, 2H), 3.37-3.42(m, 2XCH, 2H), 3.52-3.55(m, 2XCH, 2H), 3.63-3.74(m, 6XCH, 6H), 3.96(d, J=13.0Hz , 2H), 4.50(d, J=13.0Hz, 2H), 5.09(d, J=13.0Hz, 2H), 7.38-7.48(m, Ar-H, 8H), 7.62-7.65(m, Ar-H , 4H), 7.83-7.91 (m, Ar-H, 4H), 8.12-8.13 (m, Ar-H, 4H), 8.22-8.25 (m, Ar-H, 4H).
[0027] The catalyst is used to catalyze the conjugated addition rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com