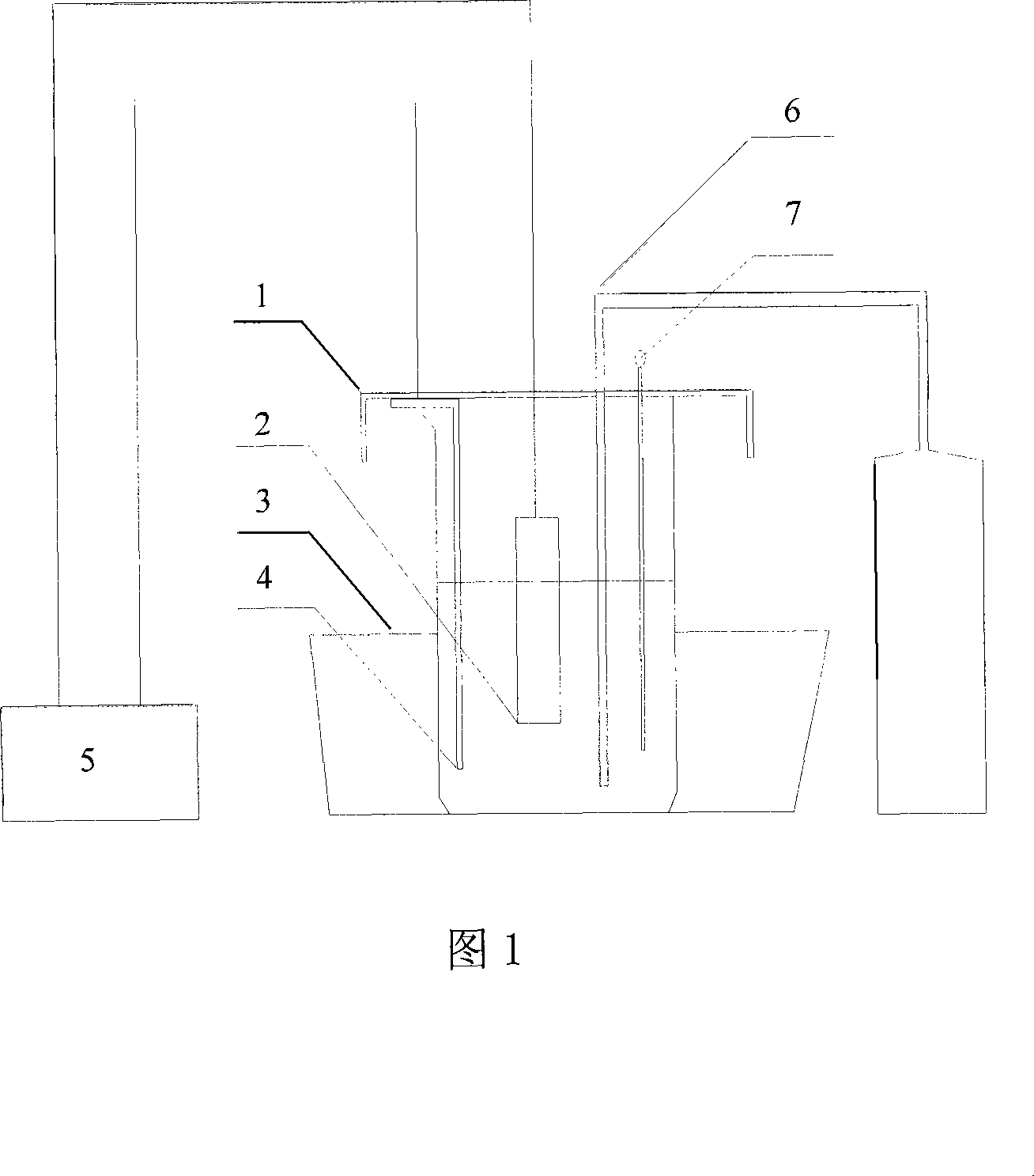Method for extracting steel superfine varia by electrolysis method
A technology of inclusion and electrolysis, applied in the field of electrochemical technology, can solve the problems of inclusion erosion, difficult non-destructive separation, etc., and achieve the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment one: first configure organic electrolyte, the formula of electrolyte is as follows: (wt%)
[0017] 2% of tetramethylammonium chloride, 8% of acetylacetone, 5% of glycerol, 6% of triethanolammonium, and methanol with a purity of 99.9% is the remainder; in addition, 5 g / L of diphenylguanidine is added by volume and weight.
[0018] Put the above electrolytic solution into the electrolytic cell; then put the steel sample containing oxide inclusions into the electrolytic cell after polishing and cleaning, as the anode, and place it at the anode position in the middle of the electrolytic cell; The control is 0.2 liters / min; the temperature of the electrolyte is controlled by an ice bath to 0~5°C; the electrolytic potential is adjusted to 2.4V, and the DC current density is 0.04A / cm 2 , electrolysis for 24 hours.
[0019] Then the above-mentioned electrolyte is further cleaned and filtered with absolute ethanol, and then put into a vacuum separation and filtration...
Embodiment 2
[0020] Embodiment 2: The organic electrolyte solution used in this embodiment is exactly the same as that of the above-mentioned embodiment 1.
[0021] In this embodiment, the steel sample containing nitride inclusions is polished and cleaned and placed in the electrolytic cell as an anode, placed in the anode position in the middle of the electrolytic cell; the inert gas argon is introduced, and its flow rate is controlled at 0.3 liters / minute ; The temperature of the electrolyte is controlled by an ice bath to 0~5°C; the electrolytic potential is adjusted to 3.2V, and the DC current density is 0.05A / cm 2 , electrolysis for 20 hours.
[0022] Then the above-mentioned electrolyte is further cleaned and filtered with absolute ethanol, and then put into a vacuum separation and filtration device with a polycarbonate membrane with a pore size of 80 nm as the filter carrier, and the ultrafine nitride inclusions are separated under vacuum operation. . Electron microscope inspectio...
Embodiment 3
[0024] The organic electrolytic solution adopted in this embodiment is exactly the same as that of the above-mentioned embodiment 1.
[0025] In this embodiment, the steel sample containing composite inclusions is polished and cleaned and placed in the electrolytic cell as an anode, placed in the anode position in the middle of the electrolytic cell; the inert gas argon is introduced, and its flow rate is controlled at 0.5 liters / min ; The temperature of the electrolyte is controlled by an ice bath to 0~5°C; the electrolytic potential is adjusted to 1.8V, and the DC current density is 0.025A / cm 2 , electrolysis for 40 hours.
[0026] Then the above-mentioned electrolyte is further cleaned and filtered with absolute ethanol and then put into a vacuum separation and filtration device with a polycarbonate membrane with a pore size of 80 nm as the filter carrier. seperate. Electron microscope inspection revealed that it was a spherical Ti-Al-O-Mn-S composite inclusion with a com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com