Detection, isolation and uses of renalase (monoamine oxidase C)
An enzyme protein, compound technology, applied in the detection, separation and application of renal enzyme (monoamine oxidase C)
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0415] The present invention includes the preparation and use of a pharmaceutical composition comprising, as an active ingredient, a compound for the treatment of arterial restenosis, adventitial fibrosis, negative remodeling, and the like. Such a pharmaceutical composition may consist of the active ingredient alone, administered to a subject in a suitable form, or the pharmaceutical composition may comprise the active ingredient together with one or more pharmaceutically acceptable carriers, one or more other ingredients or a combination of these ingredients . The active ingredient may be present in the pharmaceutical composition in the form of a physiologically acceptable ester or salt, such as in combination with a physiologically acceptable cation or anion, as is well known in the art.
[0416] As used herein, the term "pharmaceutically acceptable carrier" refers to a chemical composition combined with an active ingredient, which, after combination, is useful for administe...
Embodiment 1
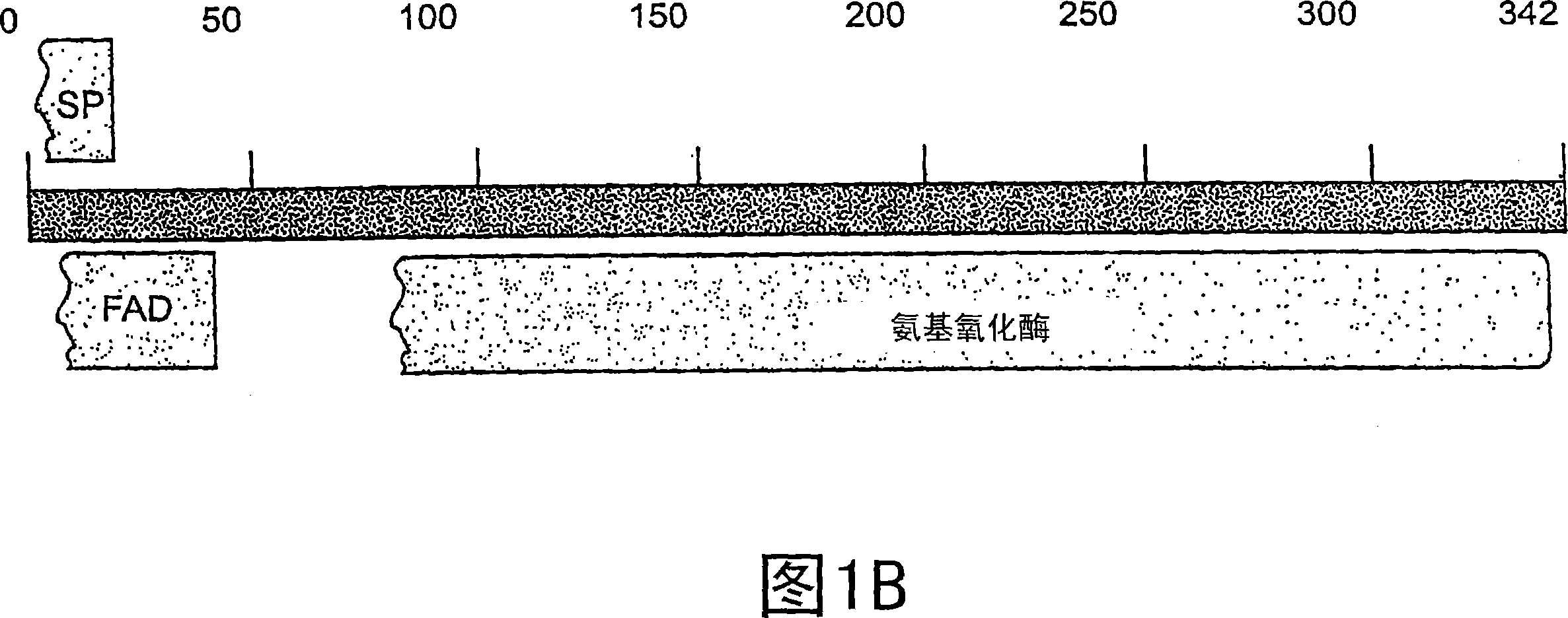
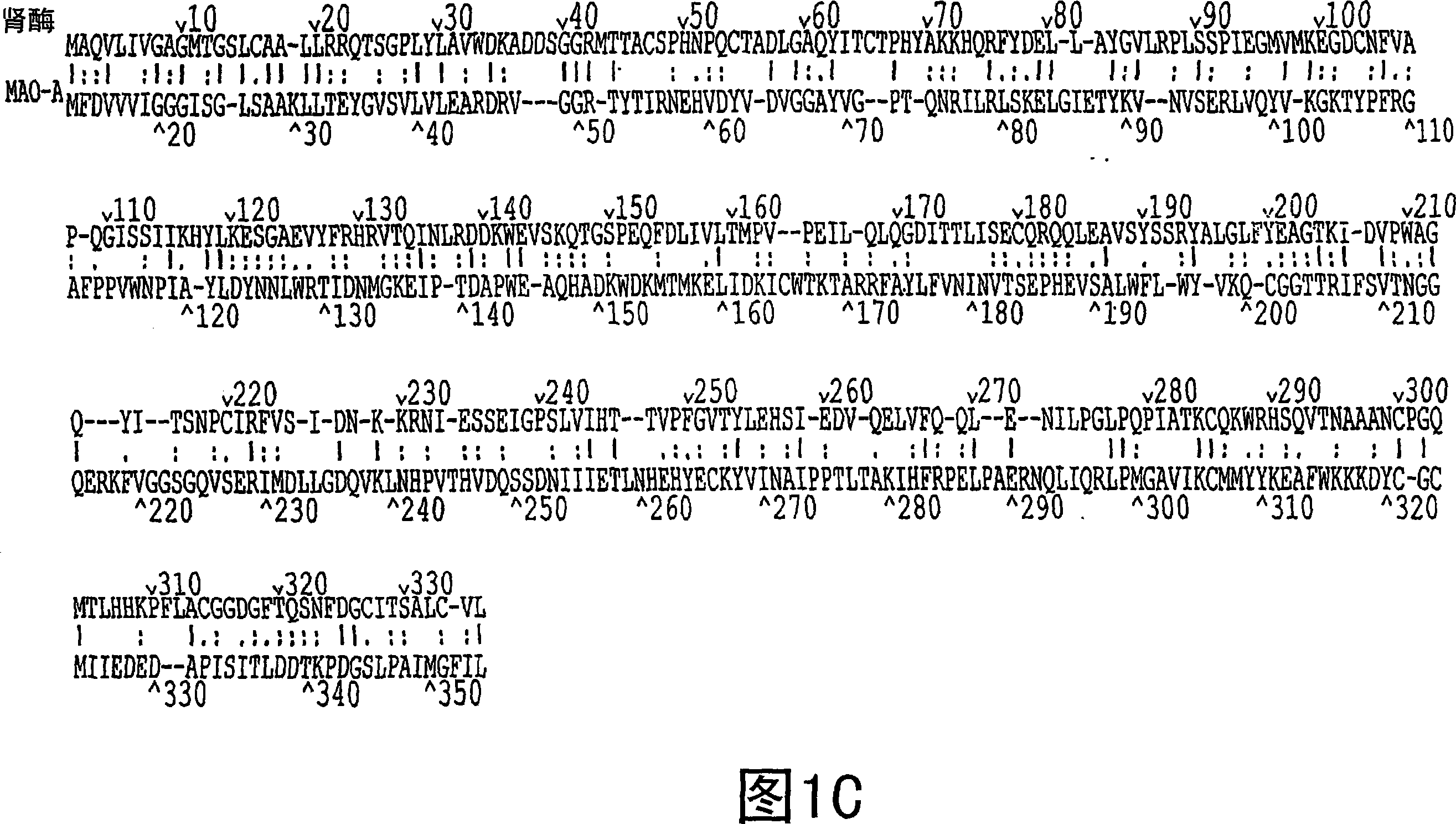
[0524] Example 1: Identification and Analysis of Genes Encoding Renal Enzymes
[0525] Materials and methods
[0526] Bioinformatics analysis of MGC database
[0527]All 12,563 unique human all-ORF cDNAs used in this experiment were obtained from the Mammalian Gene Collection Project MGC and subjected to three consecutive rounds of selection. The initial MGC analysis was performed on December 24, 2001. First, select genes without GenbankDefinition for more detailed analysis. Secondly, the predicted amino acid sequences encoded by the genes selected in the first round were analyzed using BLAST (http: / / www.ncbi.nlm.nih.gov / BLAST), and those encoding proteins with less than 20% identity to known proteins were selected. those genes of the protein. Third, the presence of putative signal sequences using SignalP V2.0 (www.cbs.dtu.dk / services / SignalP-2.0 / ) and SOSUI signal Beta_Version (http: / / sosui.proteome.bio.tuat.ac.jp )assessment. Novel proteins with signal peptide sequence...
Embodiment 2
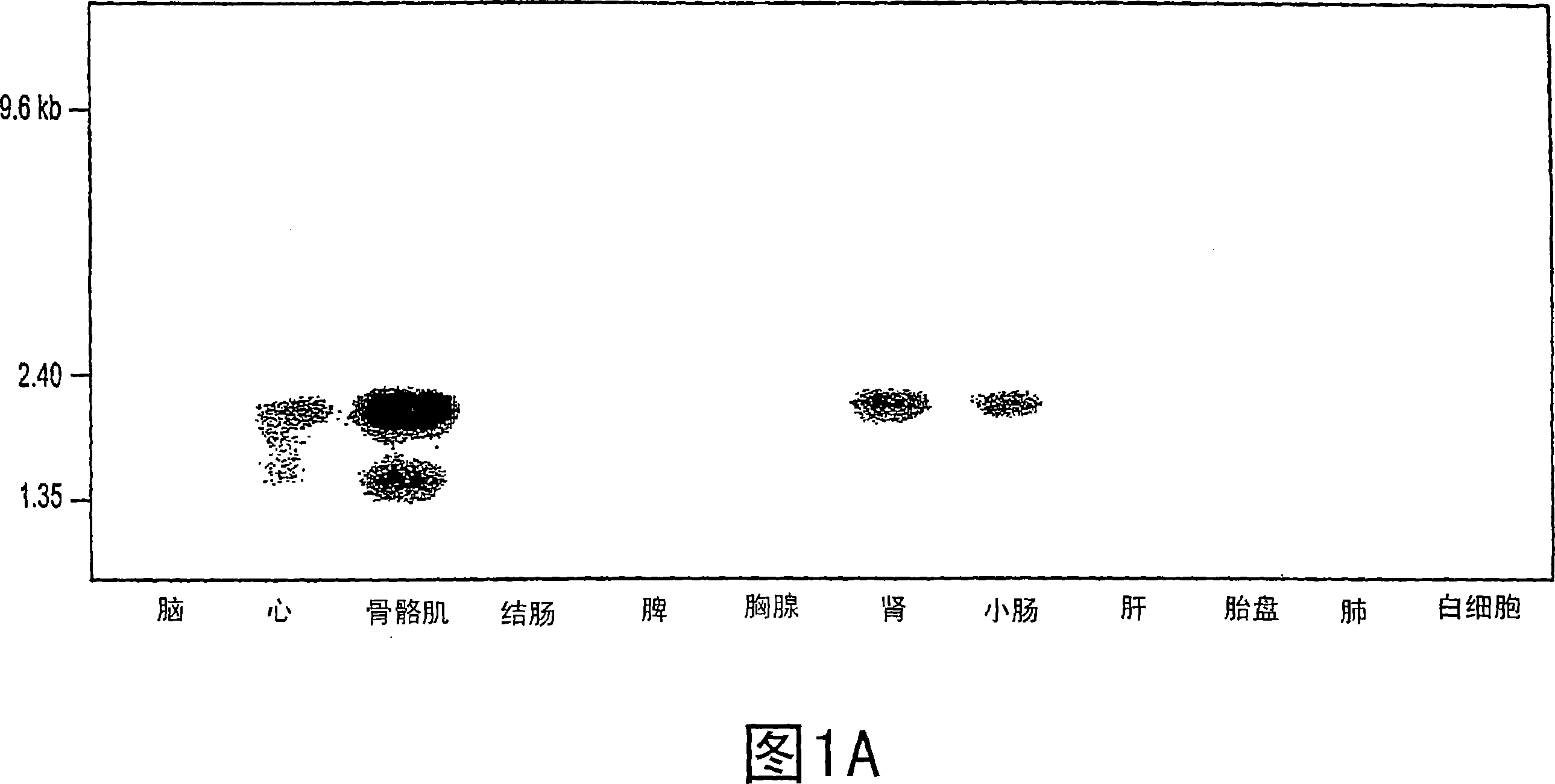
[0538] Example 2: Renal enzymes are secreted by the kidney
[0539] Construction of gene expression cassette (TAP fragment)
[0540] Using a PCR-based protocol, we added the 5′-CMV promoter and 3′-SV40pA to the 5′- and 3′-ends of each candidate clone, respectively, after two consecutive PCR reactions. The detailed methodology is described by Liang et al. Briefly, this method involves two sequential PCR steps. The first step was performed using primers (0.4 μg) containing a universal TAP end and a sequence specific for the target gene. The 5' universal end sequence was complementary to a DNA fragment containing the CMV immediate early gene promoter and was used in a second PCR step to attach the CMV promoter to the amplified gene. The 3' common end, which overlaps the DNA fragment containing the SV40 early gene transcription terminator, was also used in a second PCR step to attach the transcription terminator sequence to the amplified gene. To generate a TAP fragment contai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com