Method for determining phase change temperature of liposome
A phase transition temperature, liposome technology, applied in the field of analysis and testing, can solve the problems of complex operation and low sensitivity, and achieve the effect of avoiding interference, convenient, fast and accurate measurement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
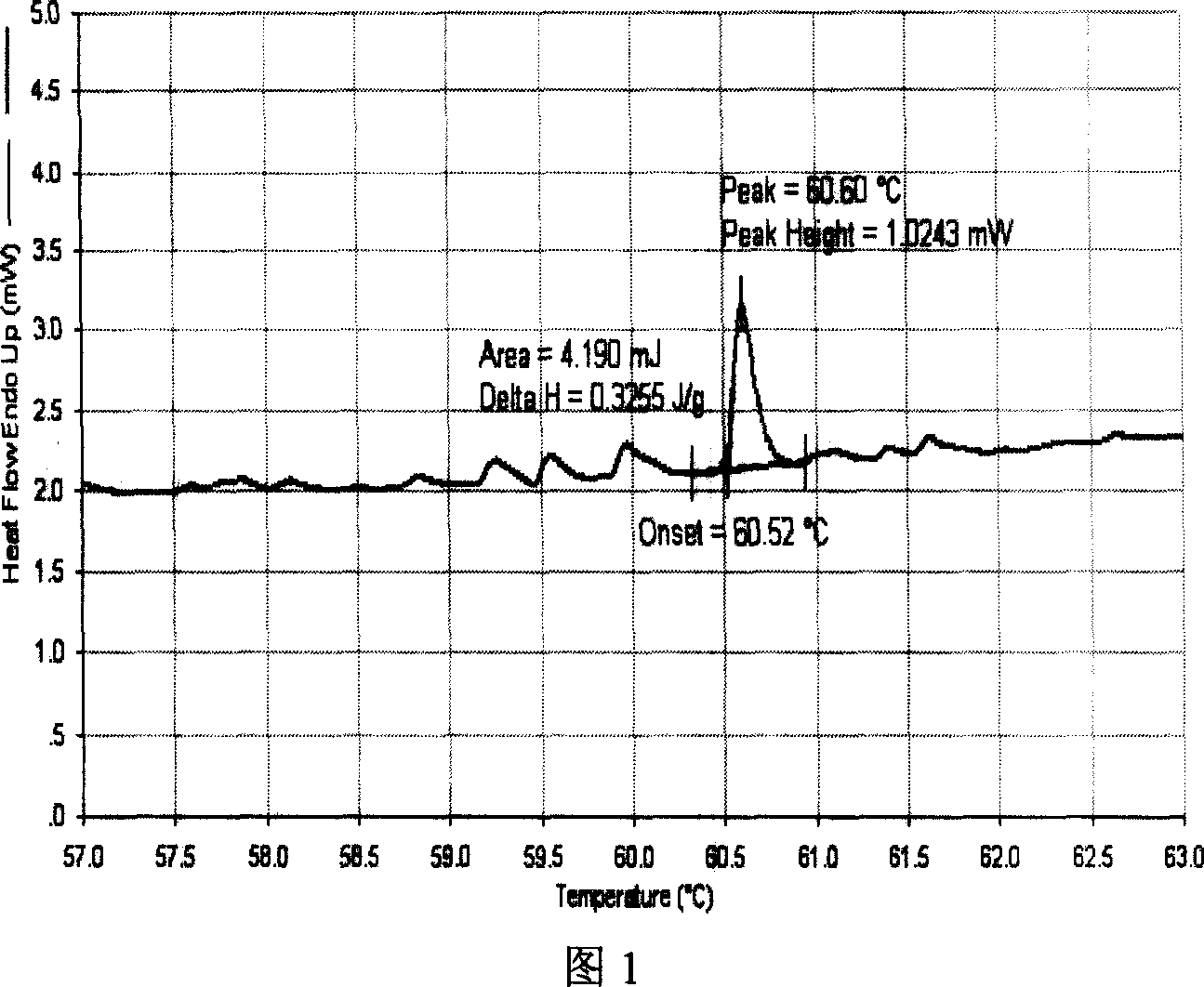
[0029] Embodiment 1: prepare berberine hydrochloride liposome (100% lecithin) suspension, the liposome suspension that will make is on centrifuge with 12000r min -1 Centrifuge for 30min, remove the supernatant, and keep the lower layer of liposomes. Weigh about 10.0 mg and place it in a differential scanning calorimeter at 2°C min -1 Heating rate detection. According to the DSC spectrum (see Figure 1), a large endothermic peak appears at 60.6°C.
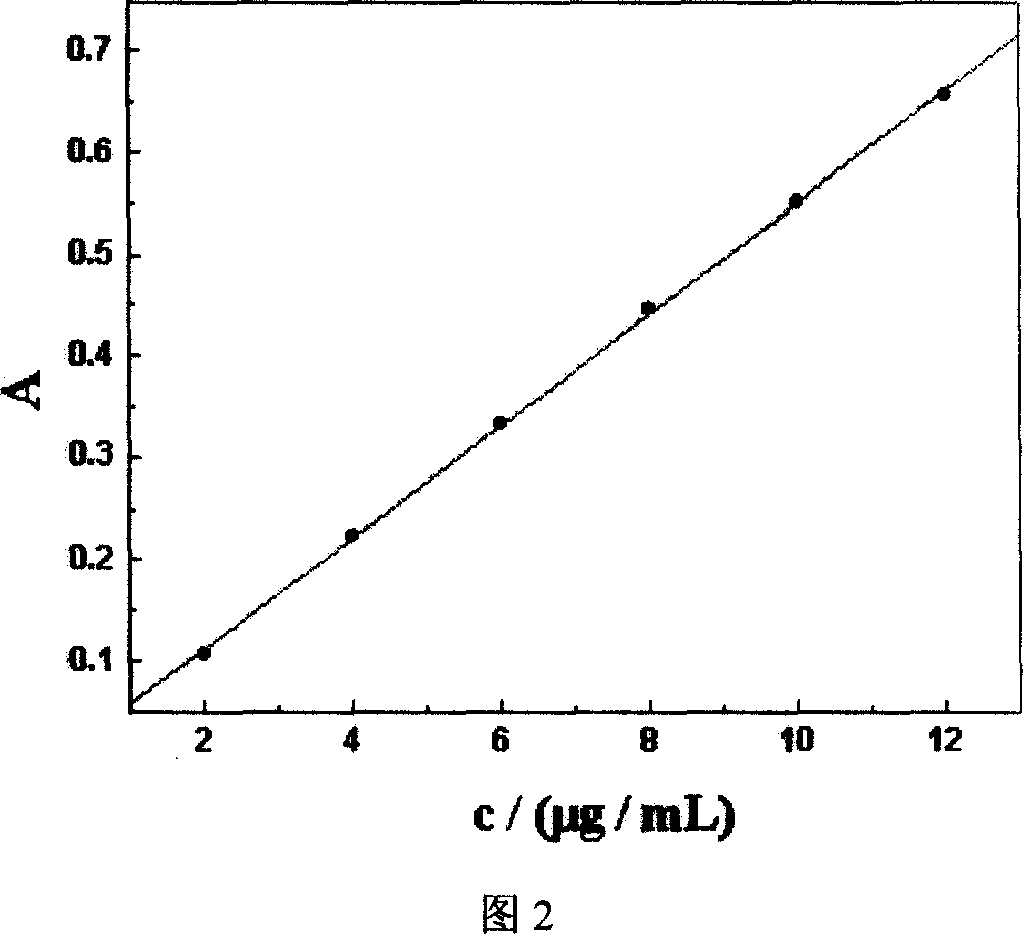
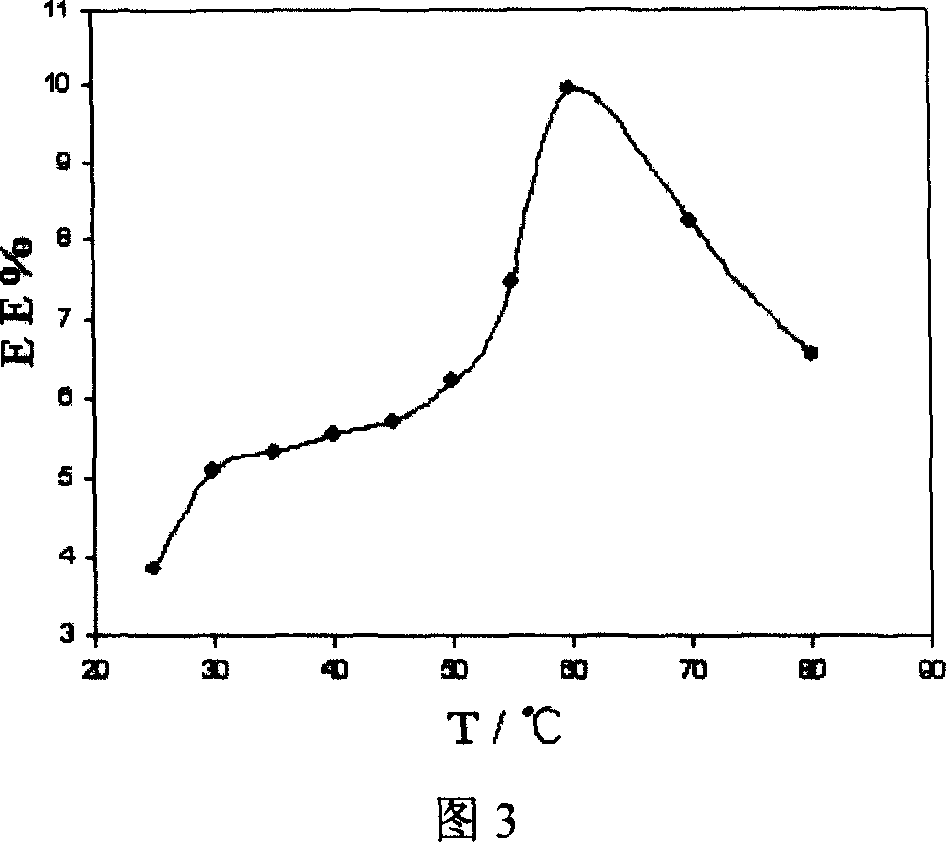
[0030] Select 25°C, 37°C, 50°C, 60°C, 70°C, and 80°C to prepare berberine hydrochloride liposomes at 6 incubation temperatures. After dialysis with a dialysis bag for 12 hours, dialyze the external fluid to a volume of 100mL. Under 345nm, measure the absorbance of solution with ultraviolet spectrophotometry, convert the concentration of solution by standard curve (see Fig. 2), the relationship between the drug encapsulation efficiency of liposome and incubation temperature is shown in Fig. 3. The results showed that the encapsulat...
Embodiment 2
[0031] Embodiment 2: prepare berberine hydrochloride liposome (lecithin and cholesterol mass ratio is 3 to 1) suspension, the liposome suspension that will make is on centrifuge with 12000r min -1 Centrifuge for 30min, remove the supernatant, and keep the lower layer of liposomes. Weigh about 10.0 mg and place it in a differential scanning calorimeter at 2°C min -1 Heating rate detection. According to the DSC spectrum (see Figure 4), a large endothermic peak appears at 41.8°C.
[0032] Select 39°C, 41°C, 43°C, and 45°C to prepare berberine hydrochloride liposomes at four incubation temperatures. After 12 hours of dialysis with a dialysis bag, dialyze the external fluid to 100mL at a constant volume, and use ultraviolet spectroscopy at 345nm. Photometer measures the absorbance of solution, and converts the concentration of solution by standard curve (see Fig. 2), and the relationship between the drug encapsulation efficiency of liposome and incubation temperature is shown in ...
Embodiment 3
[0033] Embodiment 3: prepare berberine hydrochloride liposome (lecithin and cholesterol mass ratio is 5 to 1) suspension, the liposome suspension that will make is on centrifuge with 12000r min -1 Centrifuge for 30min, remove the supernatant, and keep the lower layer of liposomes. Weigh about 10.0 mg and place it in a differential scanning calorimeter at 2°C min -1 Heating rate detection. According to the DSC spectrum (see Figure 6), a large endothermic peak appears at 55.8°C.
[0034] Select 47°C, 50°C, 53°C, 56°C, and 59°C to prepare berberine hydrochloride liposomes at 5 incubation temperatures, dialyze with a dialysis bag for 12 hours, dialyze the external fluid to 100mL at a constant volume, Measure the absorbance of the solution with a UV spectrophotometer, and convert the concentration of the solution through a standard curve (see Figure 2), and the relationship between the drug encapsulation efficiency of the liposome and the incubation temperature is shown in Figure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com