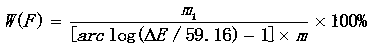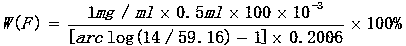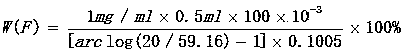Method for measuring fluorine in calcium fluoride
A measurement method, calcium fluoride technology, applied in the field of analysis and testing, can solve problems such as lowering sintering temperature, reducing the service life of production equipment, and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] Weigh 0.3999g of 1# calcium fluoride (particle size ≤ 200 mesh) to an accuracy of 0.0001, (with the sample as a reagent blank) and place it in a silver crucible filled with 6.0052 g of potassium hydroxide, cover the crucible and leave a gap, place Decompose at 680°C for 8 minutes at high temperature, take out the crucible and turn it to cool slightly, put it in a 500ml beaker, add 20-50ml hot water, cover the watch glass immediately, after the melt falls off, use hot water and a small amount of 1:5 Clean the crucible and cover with hydrochloric acid solution (scrub with a glass rod with a rubber tip). Hydrochloric acid solution was added dropwise with constant stirring until the solution was clear. Remove the beaker, rinse the watch glass and cup wall with deionized water, cool, dry filter with medium-speed filter paper and wash the acid-insoluble matter with deionized water for more than 5 times, collect the filtrate and washing solution to 500mL.
[0020] Take 10ml o...
example 2
[0025] Weigh 0.2009g of 2# calcium fluoride (particle size ≤ 200 mesh) to an accuracy of 0.0001, (with the sample as a reagent blank) and place it in a silver crucible containing 4.0031 g of potassium hydroxide, cover the crucible and leave a gap, place Decompose at 700°C for 10 minutes at high temperature, take out the crucible and turn it to cool slightly, put it in a 500ml beaker, add 20-50ml hot water, cover the watch glass immediately, after the melt falls off, use hot water and a small amount of 1:3 Clean the crucible and cover with hydrochloric acid solution (scrub with a glass rod with a rubber tip). Hydrochloric acid solution was added dropwise with constant stirring until the solution was clear. Remove the beaker, rinse the watch glass and cup wall with deionized water, cool, dry filter with medium-speed filter paper and wash the acid-insoluble matter with deionized water for more than 5 times, collect the filtrate and washing solution to 500mL.
[0026] Take 5ml of...
example 3
[0031] Weigh 0.1005g of 3# calcium fluoride (particle size ≤ 200 mesh) to an accuracy of 0.0001, (with the sample as a reagent blank) and place it in a silver crucible filled with 2.0052 g of potassium hydroxide, cover the crucible and leave a gap, place Decompose at high temperature at 720°C for 15 minutes, take out the crucible and turn it to cool slightly, put it in a 500ml beaker, add 20-50ml hot water, cover the watch glass immediately, after the melt falls off, use hot water and a small amount of 1:1 Clean the crucible and cover with hydrochloric acid solution (scrub with a glass rod with a rubber tip). Continue to add 1:1 hydrochloric acid solution dropwise under constant stirring until the solution is clear. Remove the beaker, rinse the watch glass and cup wall with deionized water, cool, dry filter with medium-speed filter paper and wash the acid-insoluble matter with deionized water for more than 5 times, collect the filtrate and washing solution to 500mL.
[0032] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com