Antibody and utilization of the same
An antibody and antibody concentration technology, applied in the direction of antibodies, anti-animal/human immunoglobulins, instruments, etc., can solve problems such as difficulty in obtaining antibodies, difficulties in antibodies, and unclear parts of essential antigens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Example 1 Preparation of a solution containing amyloid spheroids
[0108] (1) Preparation of amyloid β40 (SEQ ID NO: 1) resin
[0109]Fmoc-Val resin (342 mg, amine content: 0.73 mmol / g resin) was loaded on an A433 automatic peptide synthesizer (Perkin Elmer Applied Biosystems). Fmoc-Val-OH, Fmoc-Gly-OH, Fmoc-Gly-OH, Fmoc-Val-OH, Fmoc-Met-OH, Fmoc-Leu-OH, Fmoc-Gly-OH, Fmoc-Ile-OH, Fmoc -Ile-OH, Fmoc-Ala-OH, Fmoc-Gly-OH, Fmoc-Lys(Boc)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Gly-OH, Fmoc-Val-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Ala-OH, Fmoc-Phe-OH, Fmoc-Phe-OH, Fmoc-Val-OH, Fmoc- Leu-OH, Fmoc-Lys(Boc)-OH, Fmoc-Gln(Trt)-OH, Fmoc-His(Trt)-OH, Fmoc-His(Trt)-OH, Fmoc-Val-OH, Fmoc-Glu( OtBu)-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Gly-OH, Fmoc-Ser(tBu)-OH, Fmoc-Asp(OtBu)-OH, Fmoc-His(Trt)-OH, Fmoc-Arg (Pmc)-OH, Fmoc-Phe-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Ala-OH, and Fmoc-Asp(OtBu)-OH are added on it, using HBTU[2-(1H-benzene Triazol-1-yl)-1,1,3,3,-tetramethyluronium hexafluorop...
Embodiment 2
[0116] Example 2 Preparation of anti-amyloid spheroid antibody
[0117] (1) Preparation of rabbit anti-amyloid spheroid polyclonal antibody
[0118] The amyloid spheroids 40 and 42 prepared in Example 1 were mixed with complete Freund's adjuvant and subcutaneously administered to New Zealand white rabbits as antigens, so that 60 μg of the aforementioned amylospheroids could be administered per New Zealand white rabbit . Then, the same amount of amyloid β protein was mixed with incomplete Freund's adjuvant and administered once every 2 weeks for a total of 8 administrations. Bleeding was performed 10 days after the last immunization.
[0119] After exsanguination, the blood was allowed to remain at 37° C. for 1 hour, the resulting blood clot was removed by centrifugation, and the serum was recovered. Then, the sera were inactivated at 57°C for 30 minutes. Add ProClin300 (Sigma-Aldrich) to make the concentration 1ppm, and save. IgG is isolated from serum in the following ma...
Embodiment 3
[0127] Example 3: Analysis of Antibody Properties
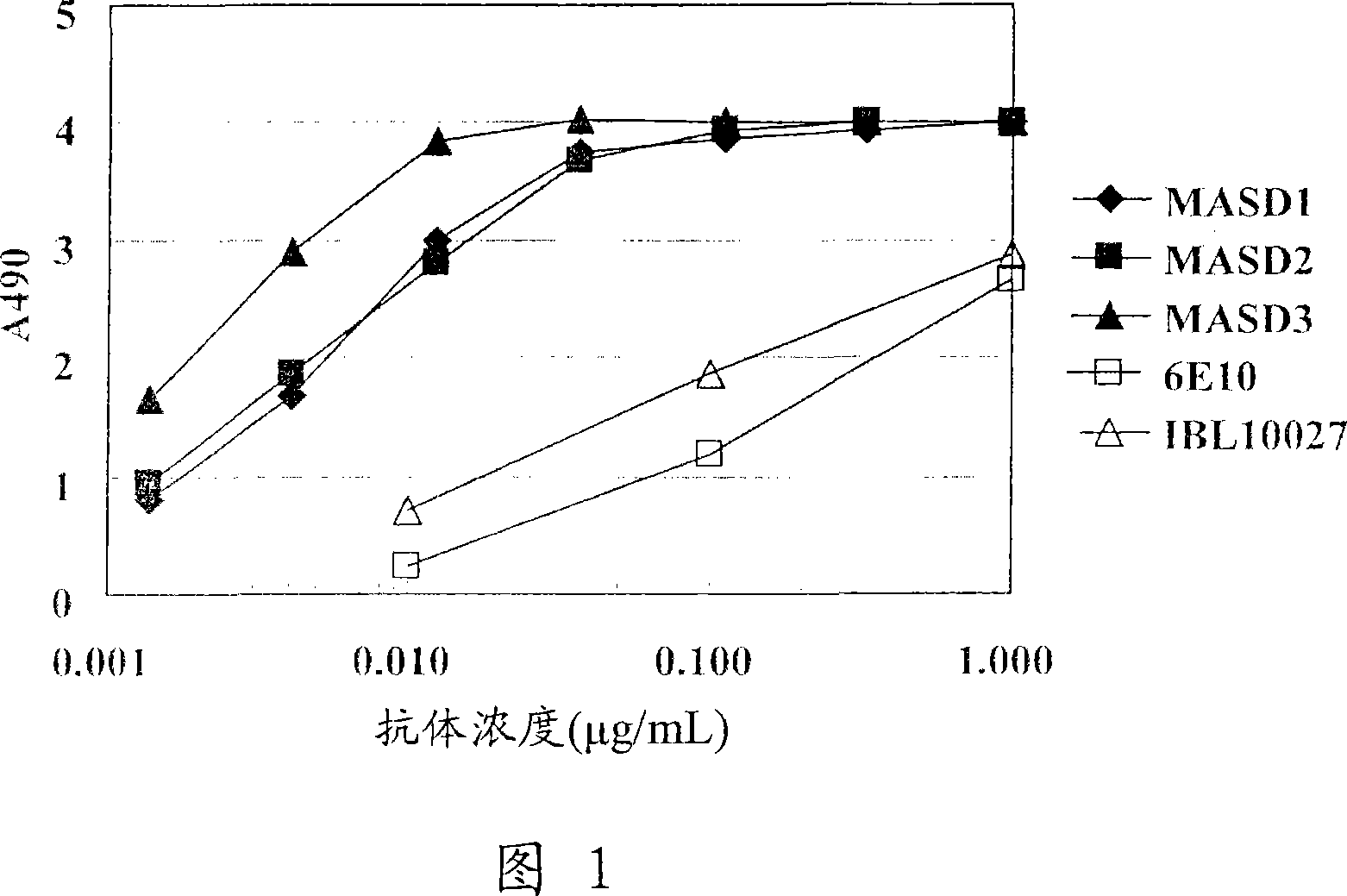
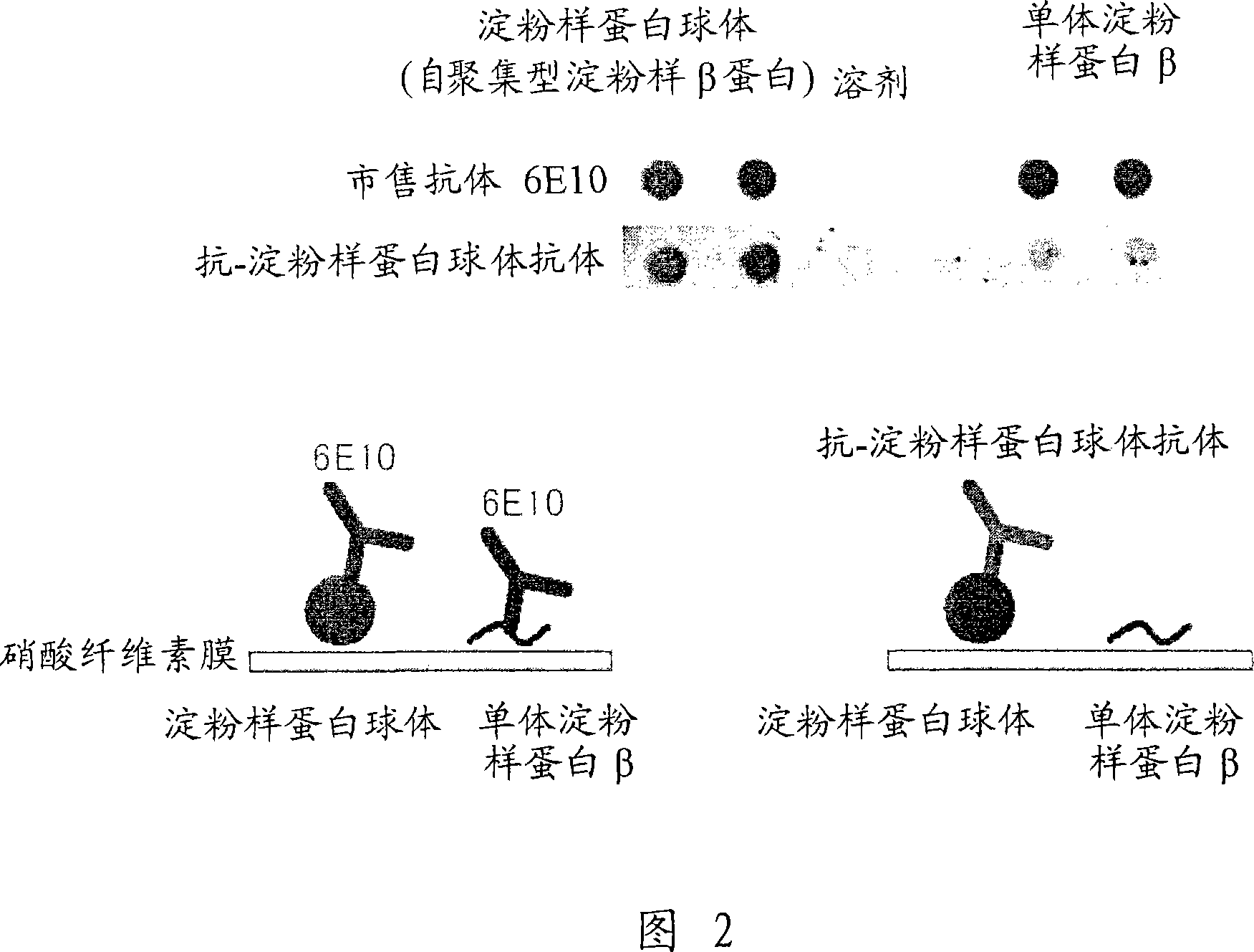
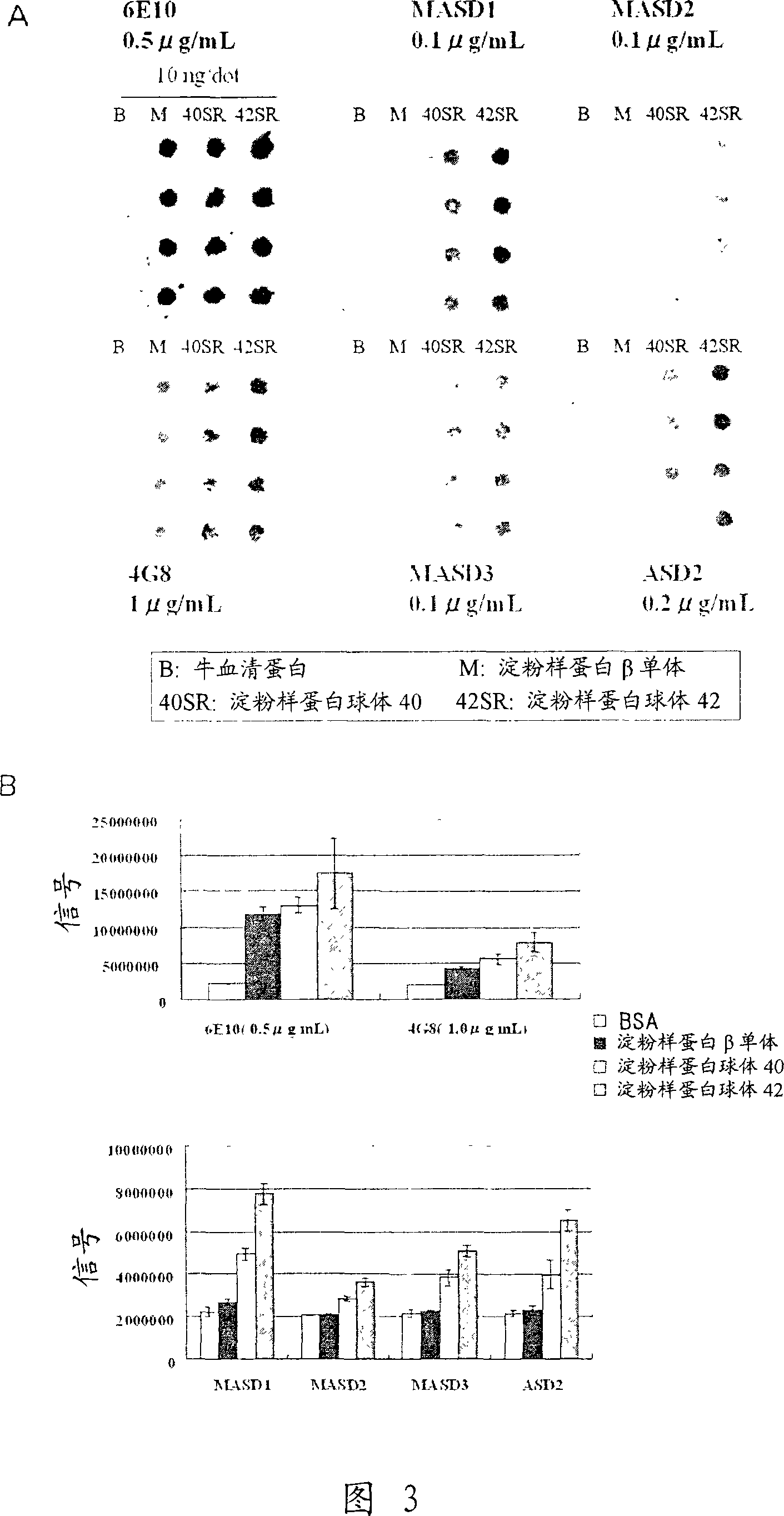
[0128] (1) Solid-phase amyloid spheroid ELISA (to determine the reactivity with amyloid spheroids)
[0129] Amyloid spheroids 40 or 42 (50 μl) diluted to 1 μg / ml in phosphate-buffered saline (without Ca or Mg, pH 7.2, PBS) were added to 96-well ELISA plates (MaxiSorp, Nunc), Plates were coated overnight at 4°C. A PBS solution containing 1% bovine serum albumin (BSA, fraction V; Sigma-Aldrich) was added thereto for at least 1 hour at room temperature to block non-specific binding sites, and the plate was washed with water. Antiserum or hybridoma culture supernatant (50 [mu]l) diluted in 1% bovine serum albumin in PBS was added and the reaction was allowed to continue for at least 1 hour at room temperature. The plate was washed 5 times with normal saline containing 0.05% Tween 20, and the peroxidase-labeled secondary antibody (anti-mouse IgG antibody (Zymed), anti-mouse IgM (Biosource), and anti-mouse immunoglobulin (DAKO))...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com