Methods for scavenging oxidizing nitrogen and oxygen species with fragrances having antioxidative properties
A scavenger, aromatic ring technology, used in gasification substances, formulations of perfume preparations, cosmetics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
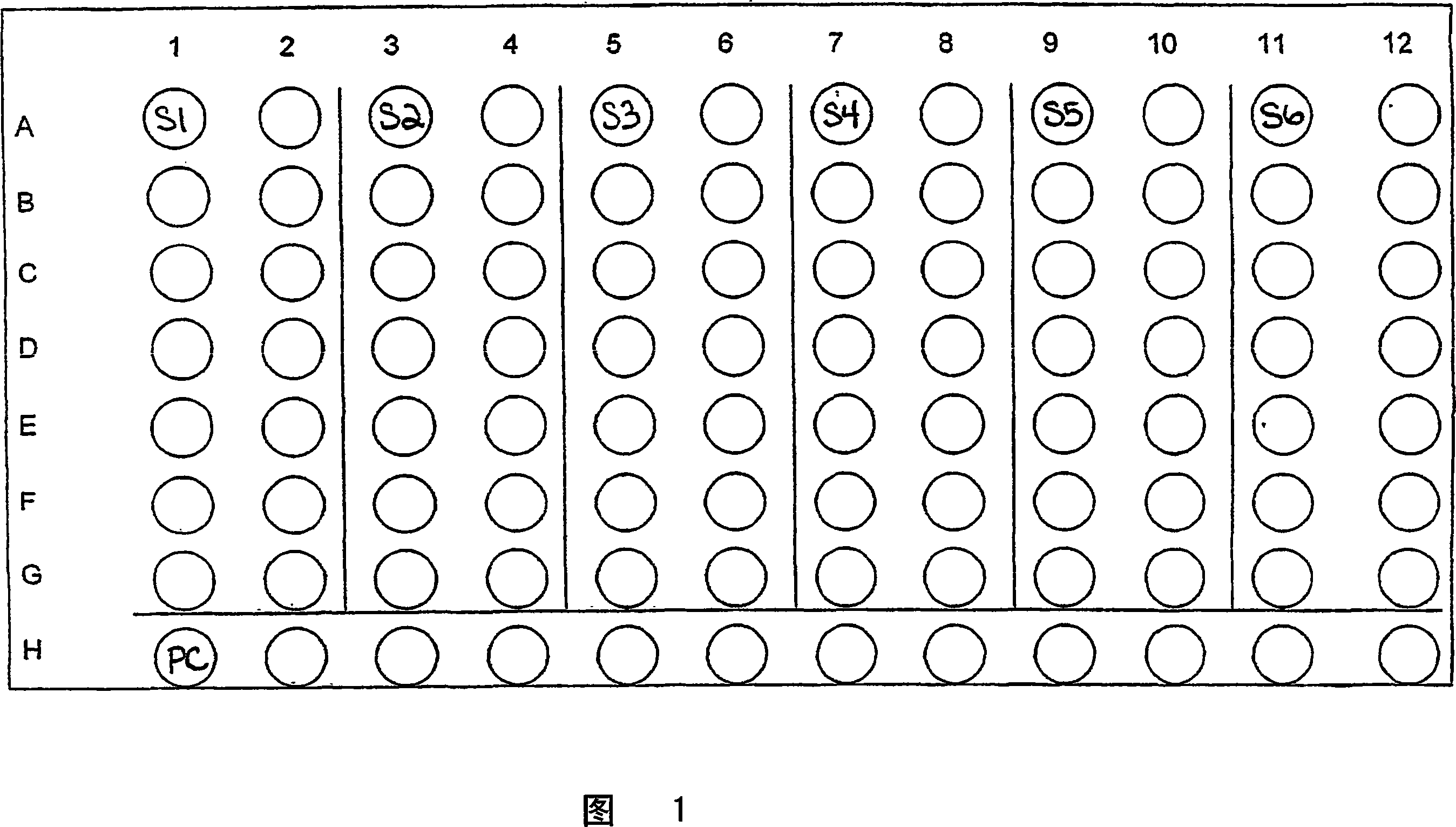
Embodiment 1
[0080] Embodiment 1: DPPH spectrophotometer analysis method
[0081] The free radical 2,2-diphenyl-1-picrylhydrazly (DPPH) can be used to determine the free radical scavenging ability of a test material such as a scavenger of the present invention. Table 2 reports the amount of various scavengers needed to consume 50% of the DPPH starting material (EC 50 ). Measurements were taken after the reaction of the scavenger being tested and DPPH had reached a steady state. DPPH are free radicals that react with antioxidants to change color. The color change of DPPH is an indication of the antioxidant capacity. Antioxidant capacity is defined as 1 / EC 50 , where EC 50 is the concentration of antioxidant required to reduce the color of DPPH by 50%. Therefore, DPPH serves as a model for the oxidative properties of free radicals. Provided below are the details for operating the DPPH spectrophotometer analysis:
[0082] Examples of scavenger tests:
[0083] - Weigh 100mg of sample ...
Embodiment 2
[0093] Example 2: Nitric Oxide Radical Absorbance Ratio (NORA) Analysis
[0094] The NORA assay is the determination of compounds that quench NO * Nitric oxide free radicals (NO * ) capabilities. In the analysis, target compounds are oxidized using the nitric oxide generator NOR-3, resulting in increased fluorescence. EC 50 . Provided below are the details for performing the Nitric Oxide Radical Absorbance Ratio (NORA) analysis:
[0095] Reagent:
[0096] Dihydrorhodamine 123, Molecular Probes (Cat * D632)
[0097] NOR-3 ((±)-(E)-ethyl-2-[(E)-hydroxyimino]-5-nitro-3-hexenamine),
[0098] Calbiochem (#489530)
[0099] Phosphate Buffer (Certified pH 7.00), Fisher (Cat#SB108-1)
[0100] N,N-Dimethylformamide, Fisher (Cat#D119)
[0101] DMSO Fisher (Cat#D136)
[0102] Methanol, HPLC pure.
[0103] Reagent preparation:
[0104] DHR123: 1.0mg / 3ml MeOH=1mM raw material (frozen and protected from light)
[0105] 7.5 μL stock solution / 10 ml buffer = 0.75 μM working solut...
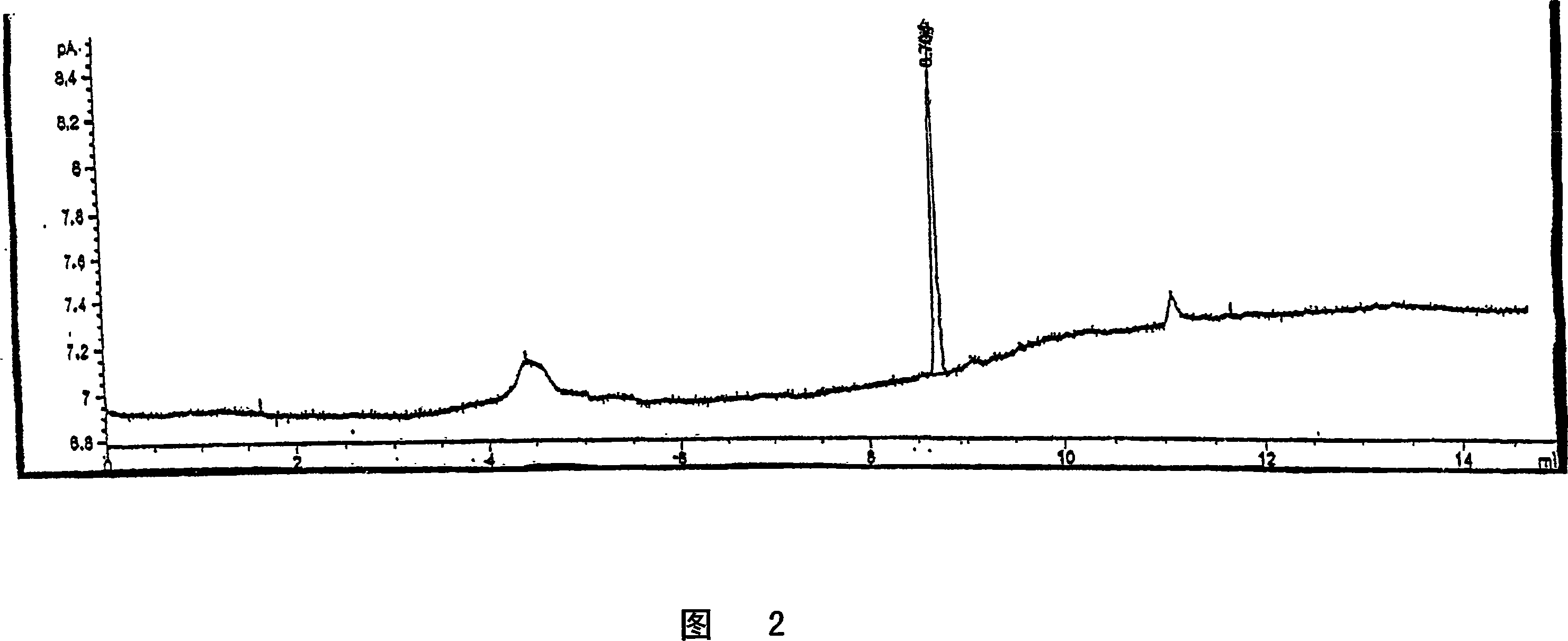
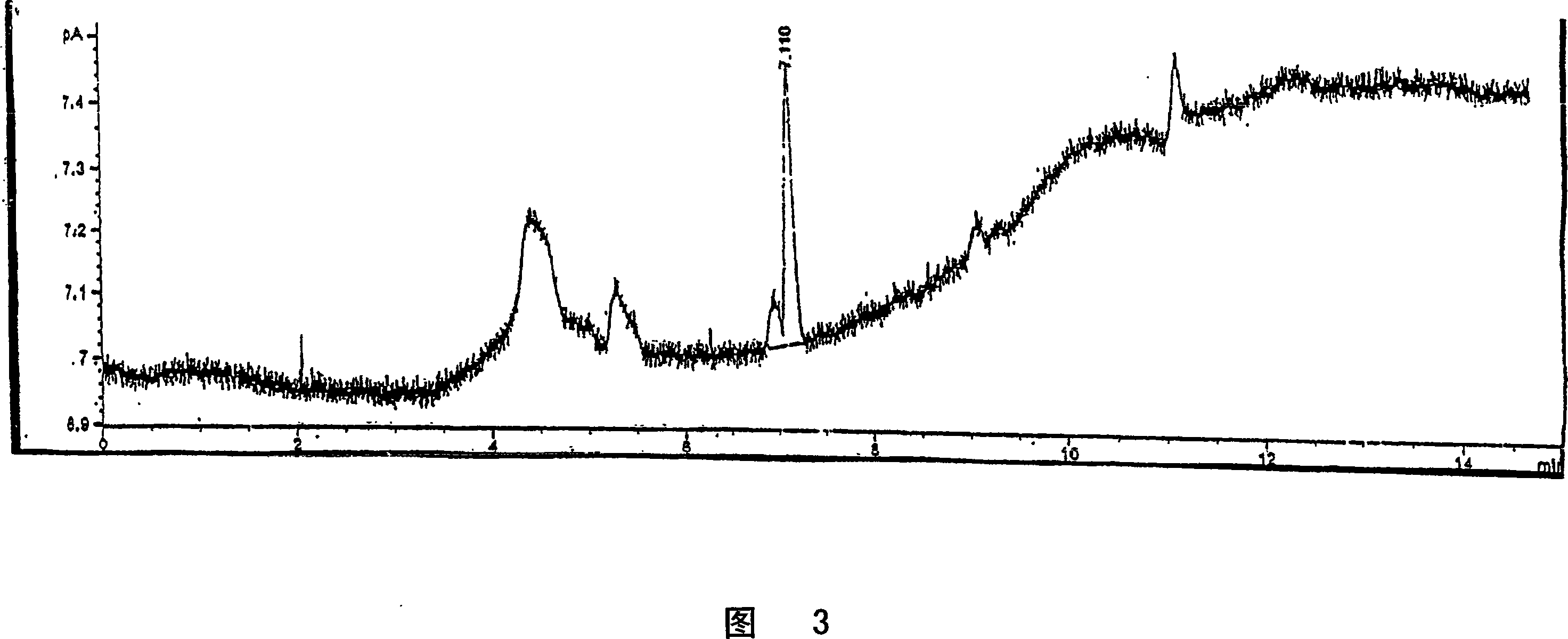
Embodiment 3
[0131] Example 3: Scanning analysis of volatile compounds
[0132] The volatility of compounds can be determined using capillary gas chromatography with flame ionization detector (GC-FID) as described below:
[0133] 1. Place approximately 1 mg of scavenger test compound or sample into a 1.5 mL glass autosampler vial fitted with a screw cap with a Teflon threaded septum. Tighten the screw cap and allow the sample to equilibrate at room temperature for 2 hours. Water was used as blank control.
[0134] 2. Place the sample vial into the autosampler of a gas chromatograph (GC) equipped with a capillary column, splitless injection, and a flame ionization detector (FID). Set the GC conditions according to the following GC methods:
[0135] GC: HP6890 or equivalent
[0136] Column: DB-I (J&W Scientific size is 25m×0.32mm film thickness 25μm) Carrier gas: Helium linear velocity is 35cm / sec (~12.4psi, 1.5mL / min)
[0137] Intake condition:
[0138] Injection: splitless
[0139] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com