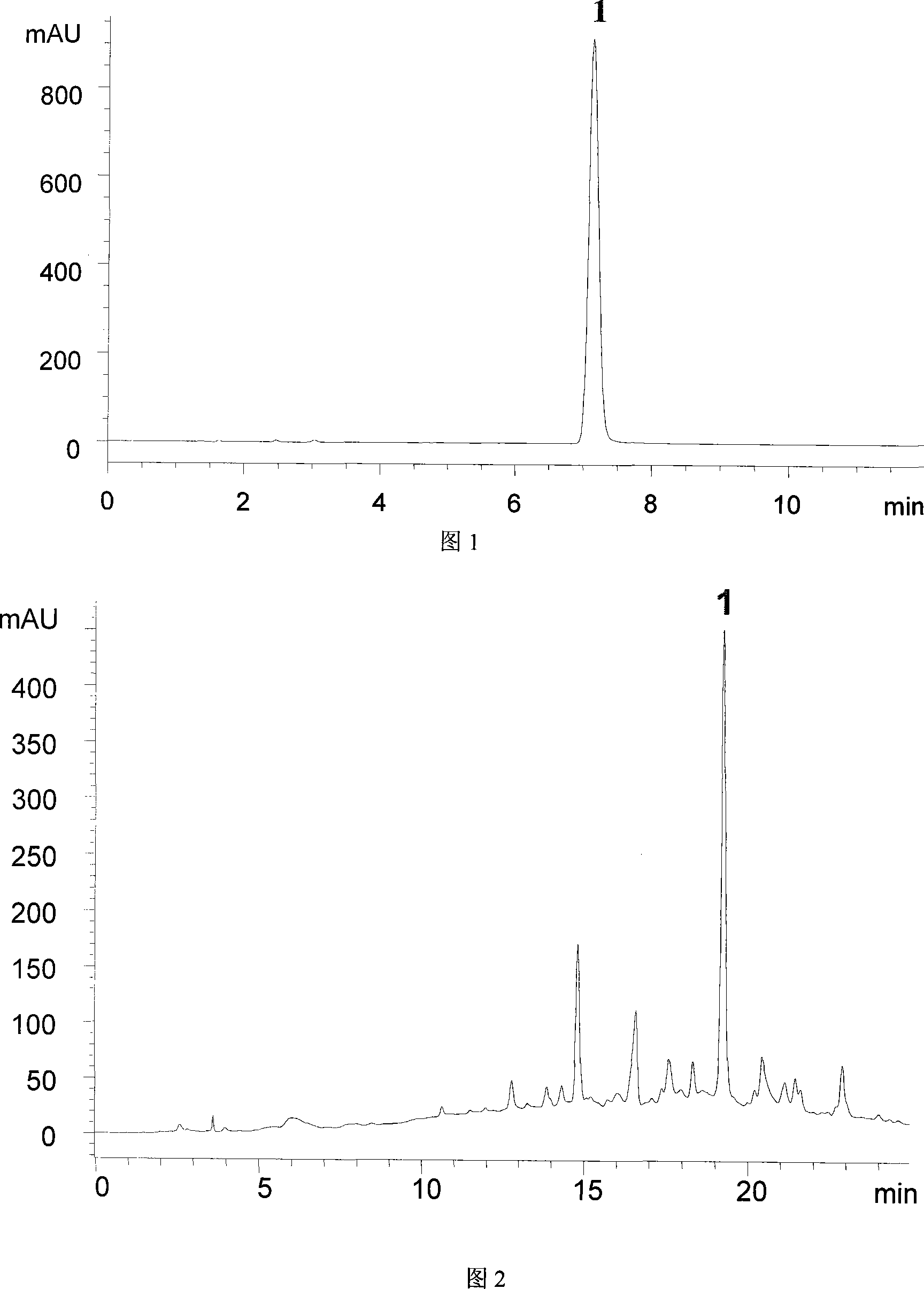Method for preparing paeonol and paeoniflorin from cortex moutan
A technology for paeonol and paeoniflorin is applied in the field of preparing paeonol and paeoniflorin step by step, which can solve the problems of limited application, waste of raw materials and the like, and achieve the effects of improving purity, low cost and reasonable design.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Take 500kg of paeonol decoction pieces, dry at 50°C for 6 hours, soak in water for 24 hours, reflux extraction, collect the distillate to 12 times the amount (6L), freeze at -4°C for 8 hours, take out and thaw and filter immediately, the insoluble matter is Dan skin phenol. Add 10 times the amount of 70% ethanol aqueous solution to the dregs, heat and reflux extraction twice in a slightly boiling state, each time for 0.5 hours, filter the extract, combine, concentrate under reduced pressure to 1g / mL (crude drug amount), and go to D 201 Macroporous resin (0.2-0.6g raw medicinal material / g wet resin). Rinse with 4BV of deionized water, discard, continue to purify with 4BV of 20% ethanol aqueous solution, discard, and then elute with 4BV of 40% ethanol aqueous solution, collect the eluate, concentrate under reduced pressure to no alcohol, and freeze-dry to obtain Paeoniae Alba glycoside part. According to liquid phase determination, the extraction rate of paeonol is 2.06...
Embodiment 2
[0028] Take 500kg of paeonol decoction pieces, dry at 50°C for 6 hours, soak in water for 10 hours, reflux extraction, collect the distillate to 8 times the amount (4L), freeze at -4°C for 4 hours, take out and thaw and filter immediately, the insoluble matter is Dan skin phenol. Add 10 times the amount of 30% ethanol aqueous solution to the dregs, heat and reflux once in a slightly boiling state, and extract once for 0.5 hours each time. The extracts are filtered, combined, concentrated under reduced pressure to 1g / mL (crude drug amount), and listed in D 201 Macroporous resin (0.2-0.6g raw medicinal material / g wet resin). Rinse with 2BV of deionized water, discard, continue to purify with 3BV of 10% ethanol aqueous solution, discard, and then elute with 3BV of 30% ethanol aqueous solution, collect the eluate, concentrate under reduced pressure to no alcohol, and freeze-dry to obtain Paeoniae Alba glycoside part. According to liquid phase determination, the extraction rate o...
Embodiment 3
[0030] Take 500kg of paeonol decoction pieces, dry at 50°C for 6 hours, soak in water for 15 hours, reflux extraction, collect the distillate to 12 times the amount (6L), freeze at -4°C for 6 hours, take out and thaw and filter immediately, the insoluble matter is Dan skin phenol. Add 8 times the amount of 50% ethanol aqueous solution to the dregs, heat and reflux in a slightly boiling state to extract twice, each time for 0.5 hours, filter the extract, combine, concentrate under reduced pressure to 1g / mL (crude drug amount), and apply to D 201 Macroporous resin (0.2-0.6g raw medicinal material / g wet resin). Rinse with 3BV of deionized water, discard, continue to purify with 3BV of 20% ethanol aqueous solution, discard, and then elute with 3BV of 50% ethanol aqueous solution, collect the eluate, concentrate under reduced pressure to no alcohol, and freeze-dry to obtain Paeoniae Alba glycoside part. According to liquid phase determination, the extraction rate of paeonol is 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com