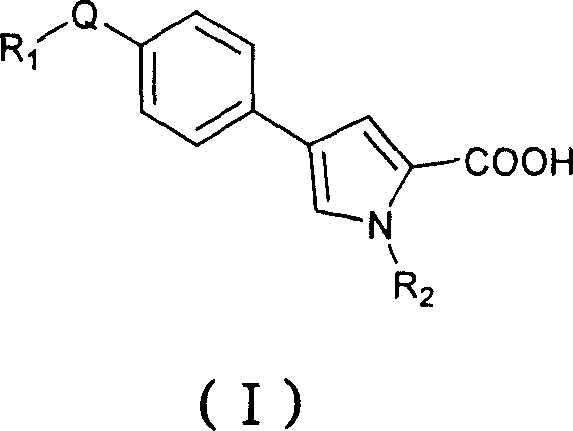
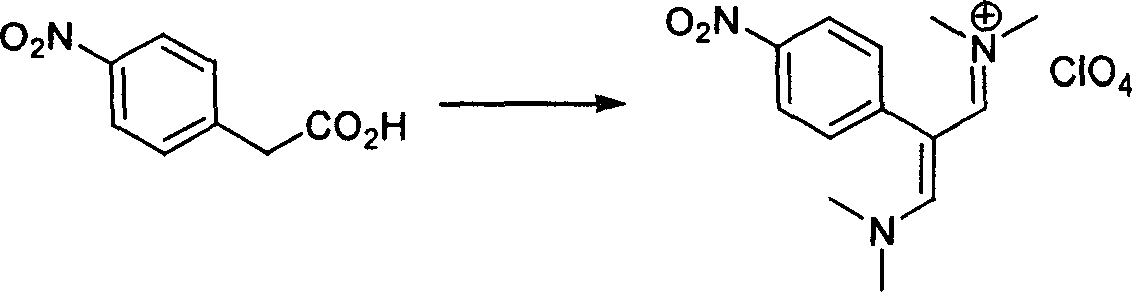Fatty acid synthetase inhibitor and application for treating bacterial infection
A technology of carboxylic acid and medicinal salt, applied in the field of pyrrole derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157] Example 1 Preparation of 1-(6-chloro-3,4-methylenedioxybenzyl)-4-(4-(2,6-difluorobenzylamino)phenyl)pyrrole-2-carboxylic acid
[0158] step 1 Preparation of 2-(4-nitrophenyl) Vinamidinium Salt
[0159] Under anhydrous conditions, 110 g (1.5 mol) of anhydrous DMF and 46 g (0.3 mol) of POCl 3 Add it into a 500ml three-necked bottle equipped with a condenser, a magnetic stirrer, and a thermometer, and react with exothermic heat, and the solution becomes colorless and black. Then add 18.1g (0.1mol) 4-nitrophenylacetic acid, heat at 70°C, and react for 7h. After the reaction finished, the reaction solution was cooled and poured into a solution that was dissolved with 15.5g (0.11mol) NaClO 4 200ml of ice-water mixture, placed in an ice bath, precipitated solid, filtered to obtain crude product, washed with a large amount of water to obtain 28.5g of product 2-(4-nitrobenzene) Vinamidinium salt, yield 82%.
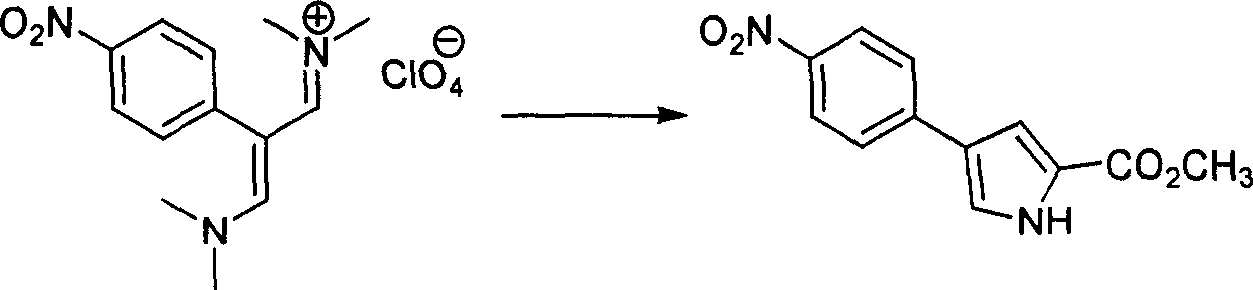
[0160] step 2 Preparation of methyl 4-(4-nitrophenyl)pyrro...
Embodiment 2
[0170] Example 2 Preparation of 1-(2-chlorobenzyl)-4-(4-(2,6-dichlorobenzylamino)phenyl)pyrrole-2-carboxylic acid
[0171] step 1 Preparation of methyl 1-(2-chlorobenzyl)-4-(4-nitrophenyl)pyrrole-2-carboxylate
[0172] According to step 3 of Example 1, 2-chlorobenzyl chloride was used instead of 6-chloro-3,4-methylenedioxybenzyl chloride to obtain 2.41 g of a yellow solid product with a yield of 65.1%. 1 H-NMR (d 6 -DMSO) δ: 3.71(s, 3H), 5.69(s, 2H), 6.50(dd, J=7.6Hz, 2Hz, 1H), 7.24-7.32(m, 2H), 7.51(dd, J=7.8Hz , 1.4Hz, 1H), 7.59(d, J=2Hz, 1H), 7.91(d, J=8.7Hz, 2H), 8.03(d, J=2Hz, 1H), 8.19(d, J=8.7Hz, 2H).
[0173] step 2 Preparation of methyl 1-(2-chlorobenzyl)-4-(4-aminophenyl)pyrrole-2-carboxylate
[0174] According to step 4 of Example 1, replace 1-(6-chloro-3,4-methylene with 1-(2-chlorobenzyl)-4-(4-nitrophenyl)pyrrole-2-carboxylic acid methyl ester Dioxybenzyl)-4-(4-nitrophenyl)pyrrole-2-carboxylic acid methyl ester to obtain the product as a brown solid ...
Embodiment 3
[0179] Example 3 Preparation of 1-(3-chlorobenzyl)-4-(4-(2,6-dichlorobenzylamino)phenyl)pyrrole-2-carboxylic acid
[0180] step 1 Preparation of methyl 1-(3-chlorobenzyl)-4-(4-nitrophenyl)pyrrole-2-carboxylate
[0181] According to step 3 of Example 1, 3-chlorobenzyl chloride was used instead of 6-chloro-3,4-methylenedioxybenzyl chloride to obtain 2.37 g of a yellow solid product with a yield of 64.1%. 1 H-NMR (d 6 -DMSO) δ: 3.75(s, 3H), 5.59(s, 2H), 7.12(d, J=7Hz, 1H), 7.25(s, 1H), 7.33-7.39(m, 2H), 7.53(d, J=2Hz, 1H), 7.91(d, J=8.7Hz, 2H), 8.13(d, J=2Hz, 1H), 8.20(d, J=8.7Hz, 2H).
[0182] step 2 Preparation of methyl 1-(3-chlorobenzyl)-4-(4-aminophenyl)pyrrole-2-carboxylate
[0183] According to step 4 of Example 1, replace 1-(6-chloro-3,4-methylene with 1-(3-chlorobenzyl)-4-(4-nitrophenyl)pyrrole-2-carboxylic acid methyl ester Dioxybenzyl)-4-(4-nitrophenyl)pyrrole-2-carboxylic acid methyl ester to obtain the product as a brown solid with a yield of 55.9%. 1 H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com