Sphingosine 1-phosphate agonists comprising cycloalkanes and 5-membered heterocycles substitued by amino and phenyl groups
A cycloalkyl, alkyl technology, applied in the field of novel sphingosine-1-phosphate analogues, which can solve problems such as limitations, lack of solubility, and synthetic complexity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
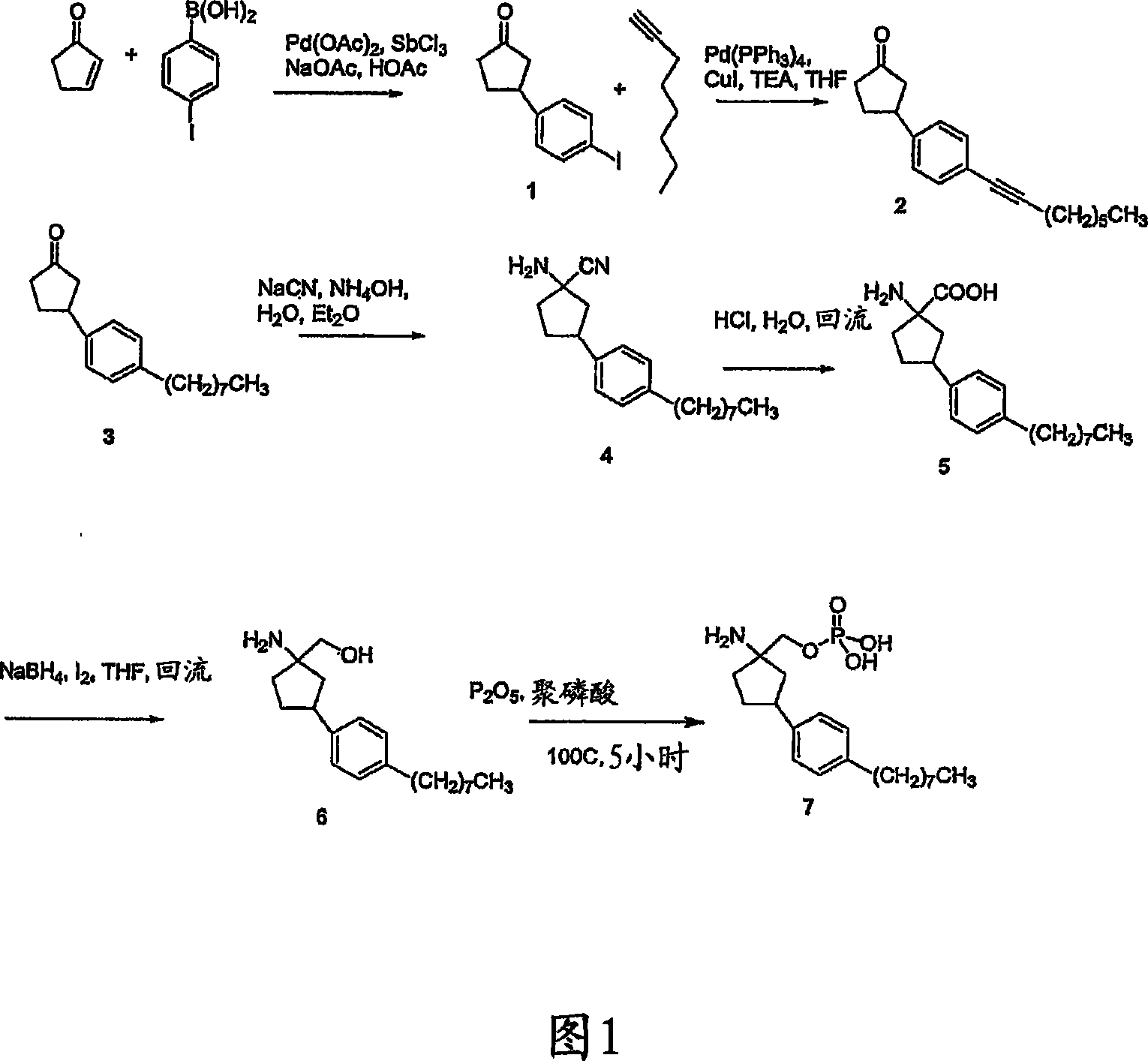
[0206] Example 1: (1-amino-3-(4-octylphenyl)cyclopentyl)methanol (6)
[0207] A: 3-(4-iodophenyl)cyclopentanone (1)
[0208] in N 2 0.23 g palladium(II) acetate (0.1 eq) and 0.23 g antimony (III) chloride (0.1 eq) were added to 80 mL containing 0.82 g (10 mmol) 2-cyclopent-1-one, 2.48 g (10 mmol) Acetic acid solution of 4-iodophenylboronic acid and 1.6g (20mmol) sodium acetate. After stirring at 25°C for 24 hours, the black precipitate was removed by filtration and the filtrate was diluted with 250 mL of brine and extracted twice with 50 mL of dichloromethane. Use saturated NaHCO 3 The solution was stirred with the organic extract for 30 min, then washed with brine and washed with MgSO 4 dry. Removal of solvent gave a yellow oil which was further purified using flash column (chloroform) to give 1.92 g (67%) of the product as a white solid. J. Org. Chem., 1995, 60, 883-888.
[0209] 1 H NMR (CDCl 3 )δ7.63 (d, 2H, ArH), 7.00 (d, 2H, ArH), 3.35 (m, 1H, ArCHCC), 2.7-1.8...
Embodiment 2
[0229] Example 2: (1-amino-3-(4-octylphenyl) cyclopentyl) methyl dihydrogen phosphate (7)
[0230] (1-Amino-3-(4-octylphenyl)cyclopentyl)methyl dihydrogen phosphate (7)
[0231] 1 mL of 85% H 3 PO 4 Slowly add 0.5 g of P 2 o 5 , in, and then at N 2 Under protection, the acid-anhydride mixture was heated at 100°C for 1 hour. Add another 0.5g of P 2 o 5 and 30mg of 6 were added to polyphosphoric acid and heated at 100°C for 5 hours. After cooling to room temperature, 10 mL of ice water was added to the reaction mixture. The product precipitated out as a white solid. The product was collected and washed with water. 31 mg (82%) of the green product were collected after vacuum drying. MS only two peaks: M+1 = 384.4 and 304.4 (hydrolyzed back to 6).
Embodiment 3
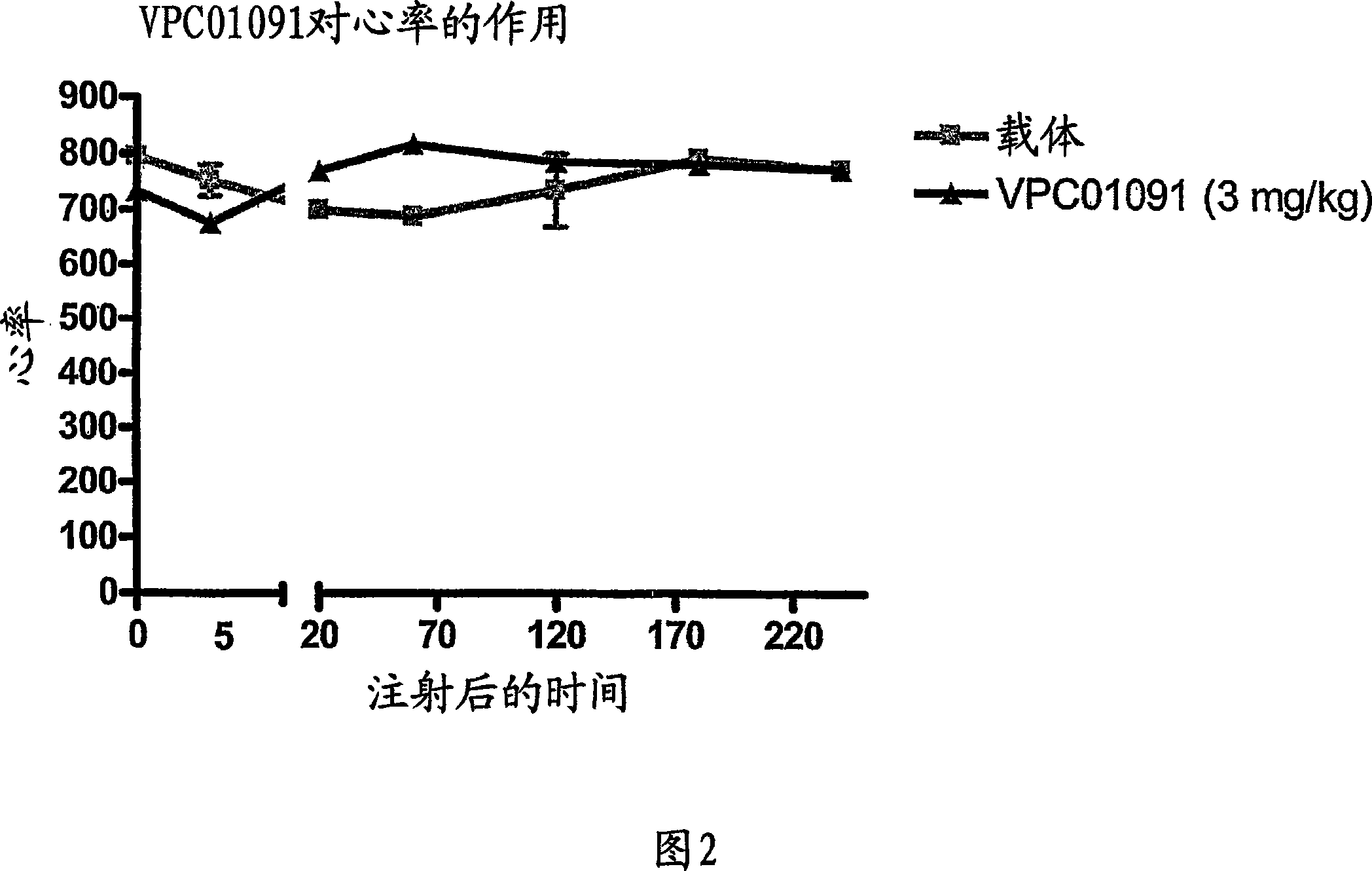
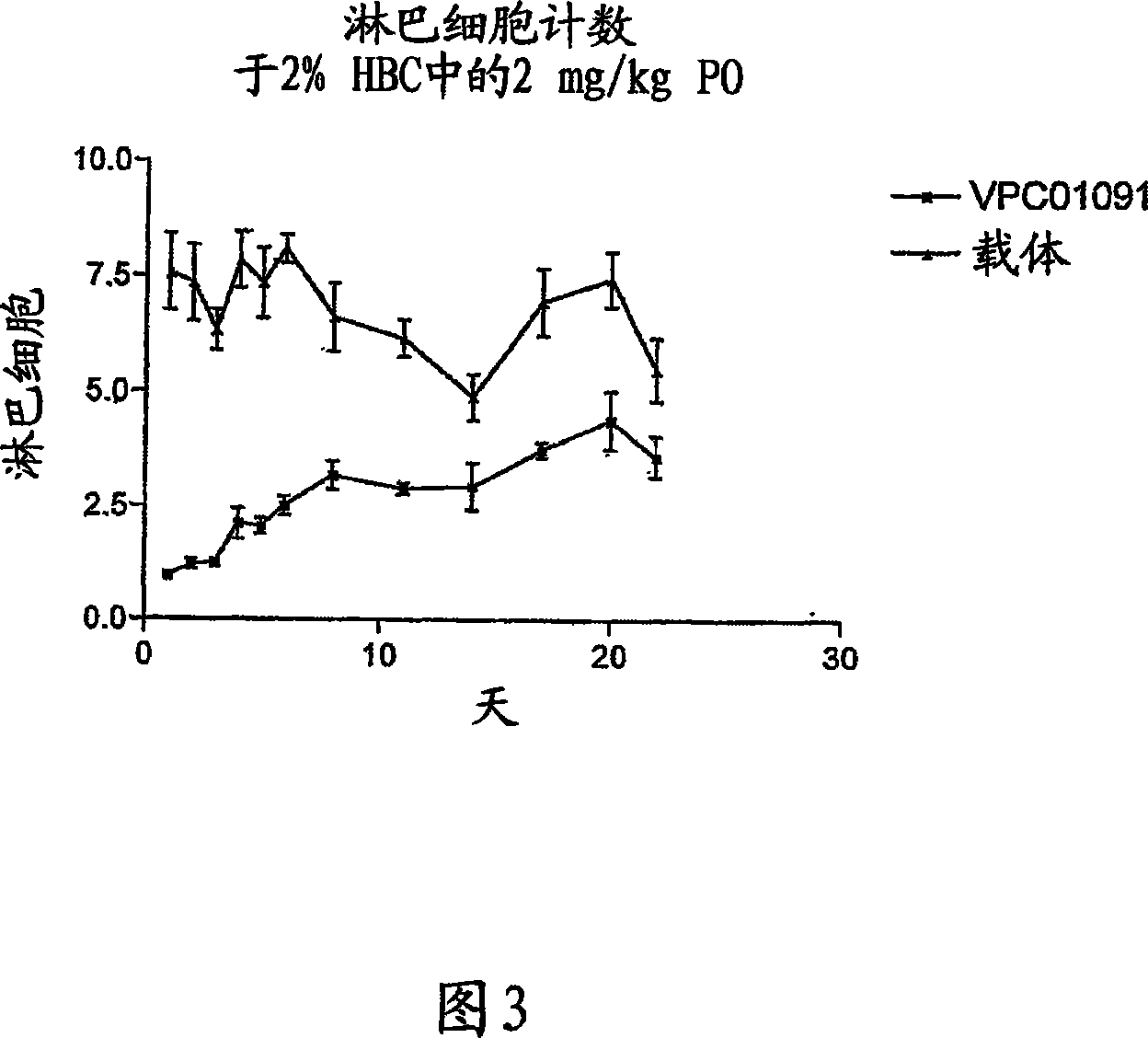
[0232] Example 3: GTPγS-35 binding assay
[0233] This assay demonstrates agonist activity of G protein-coupled receptors (GPCRs) in isolation. This assay induces recombinant GPCRs (such as S1P1-5 receptors) and three subunits of heterotrimeric G proteins (typically α1, β2, γ3) by transfecting HEK293T cells with four plasmid DNAs encoding each protein Concomitant expression of each in said cells. Approximately 60 hours after transfection, cells were harvested, disrupted, and nuclei discarded. This allows the preparation of coarse microsomes from the residue. Stimulation with agonists (eg, S1P) of the G protein receptor complex on microsomes results in a dose-dependent conversion of GTP to GDP on the alpha subunit. To detect the GTP-bound alpha subunit, a GTP analog (GTPγS-35), a radionuclide (sulfur-35) labeled phosphorothioate that is not hydrolyzed to GDP, was used. Microsomes with attached G protein were collected by filtration and bound GTPγS-35 was quantified in a l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com