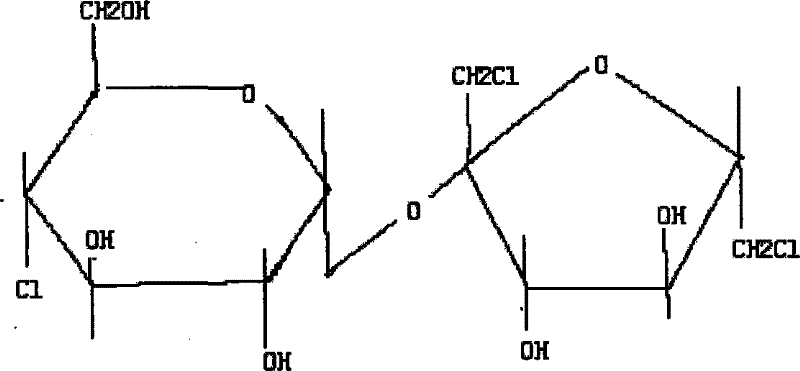
Method of preparing sucralose
A technology for sucralose and sucrose, which is applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of residue pollution, difficult preparation, and difficult to recover, and achieves low production cost and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0023] Sucrose 34.2g (0.1mol) DMF 200ml, warm up to complete dissolution, cool, add p-toluenesulfonic acid 0.5g and vinyl acetate 9.7g (0.112mol), stir at room temperature for 12 hours overnight. TLC showed that there was almost no sucrose, vinyl acetate and by-product acetaldehyde were removed under reduced pressure, pH was adjusted to 9-9.5 with tert-butylamine, the temperature was raised to 40°C and kept for 2 hours, DMF was evaporated under reduced pressure to obtain 52.8 g of syrup, and the measured sucrose- The content of 6-acetate is 64.5%, and the yield is 88.7%.
Embodiment 3
[0025] Sucrose 171g (0.5mol) DMF 1000ml, in a 2000ml four-neck bottle, heated to complete dissolution, cooled to room temperature, added 1.0g benzenesulfonic acid and vinyl acetate 48.60g (0.565mol), stirred at room temperature overnight, TLC showed almost no sucrose, Remove excess raw materials and by-products under reduced pressure, adjust the pH to 9-9.5 with tert-butylamine, raise the temperature at 40°C and keep for 2 hours, evaporate DMF under reduced pressure to obtain 267.8 g of syrup, and the content of sucrose-6-acetate was measured to be 62.7%. Yield 87.4%.
Embodiment 4
[0027] Sucrose 3.42g (0.01mol), DMF 30ml, heat up to dissolve, cool to room temperature, add toluene solution containing 0.1g of dibutyltin oxide chloride and 1.7g (0.0105mol) of isopropenyl benzoate, stir overnight or track hexyl sugar by TLC Basically disappear, remove acetone under reduced pressure, add tert-butylamine to adjust pH to 9~9.5, raise temperature at 40°C for 2 hours, evaporate DMF under reduced pressure, add 30ml of acetone, stir and reflux for 1 hour, filter, wash with a small amount of acetone, reduce Dry under pressure to obtain 4.3 g of sucrose-6-benzoate, with a content of 98.7% and a yield of 94.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com