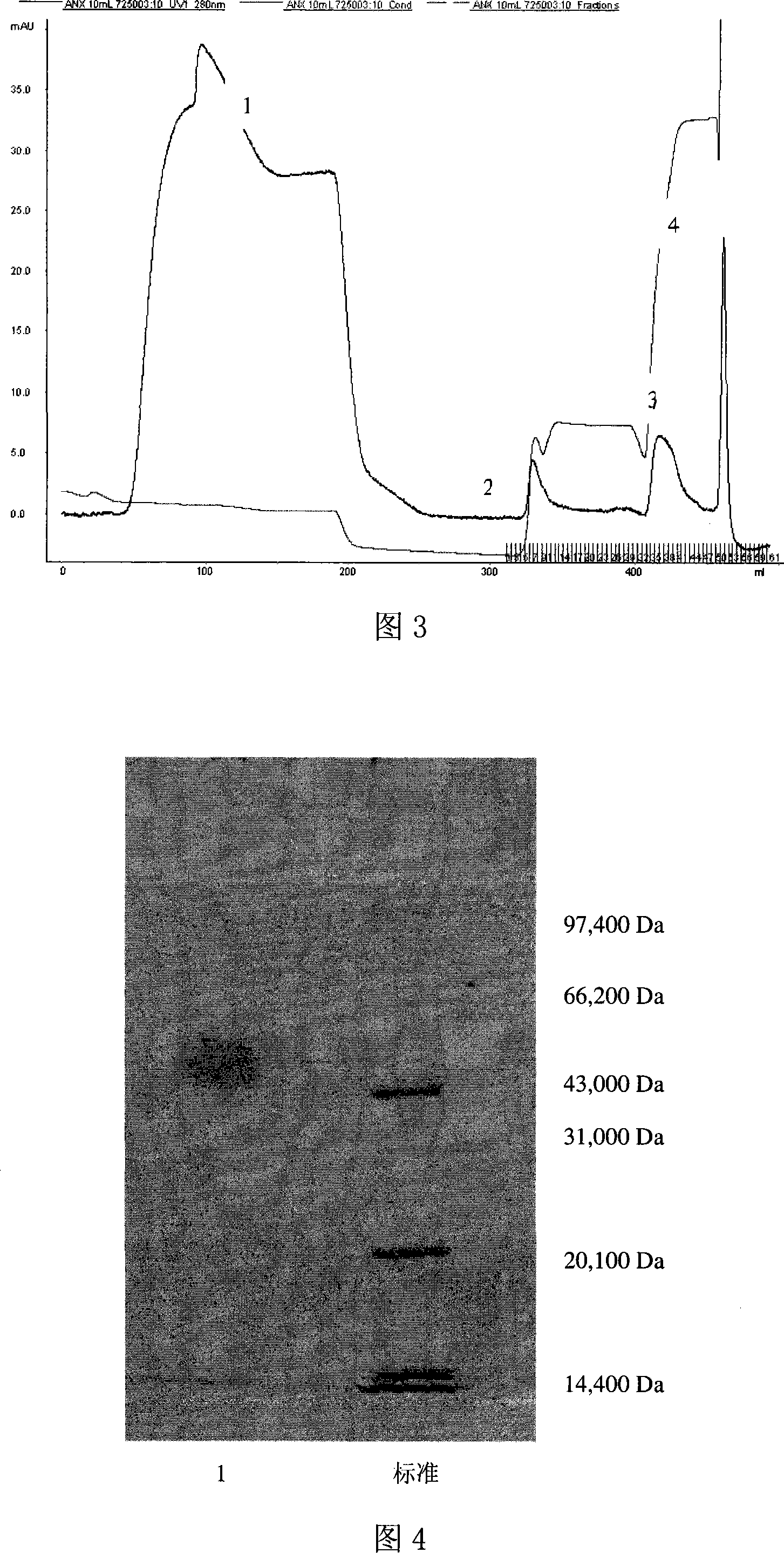
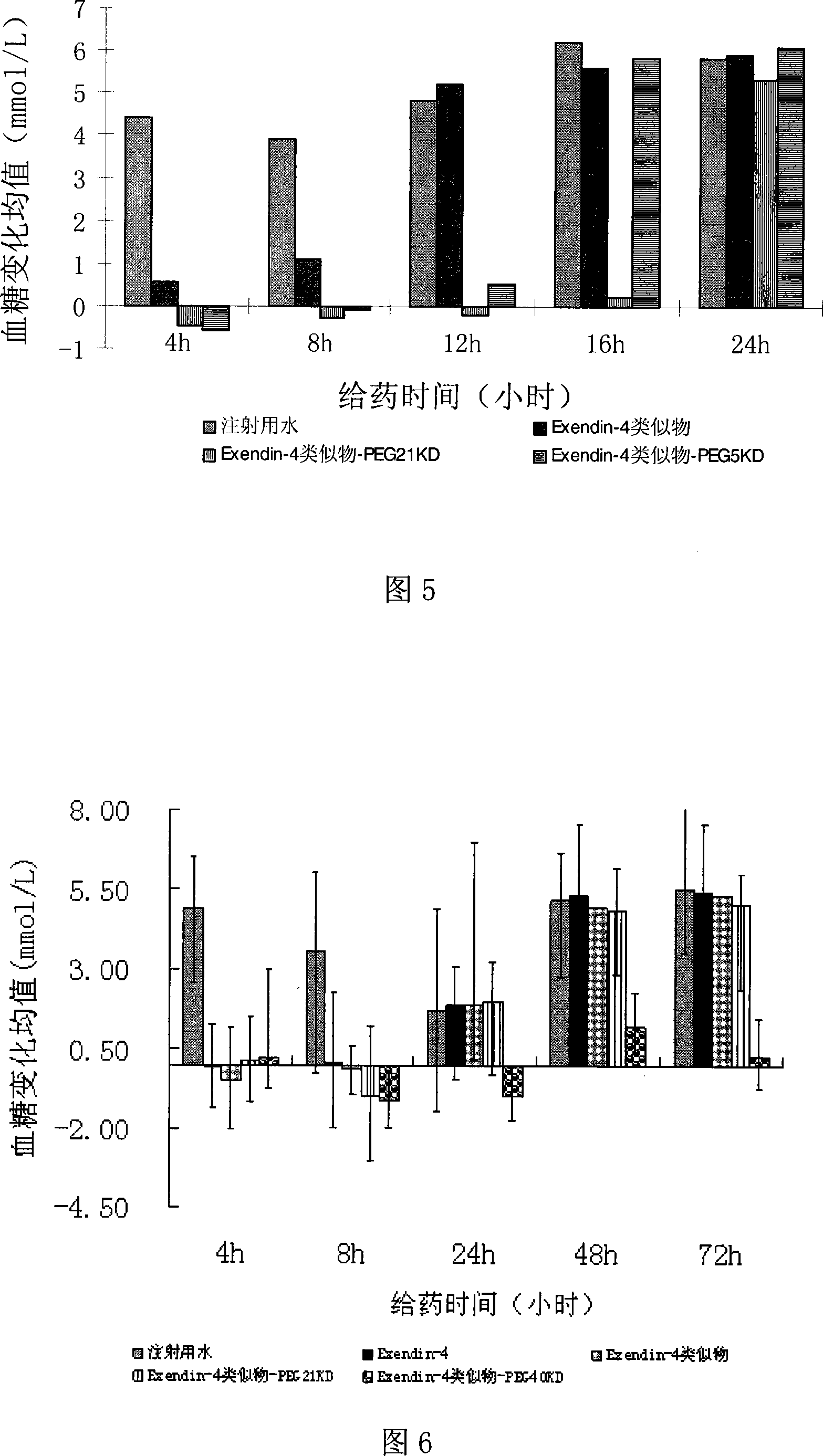
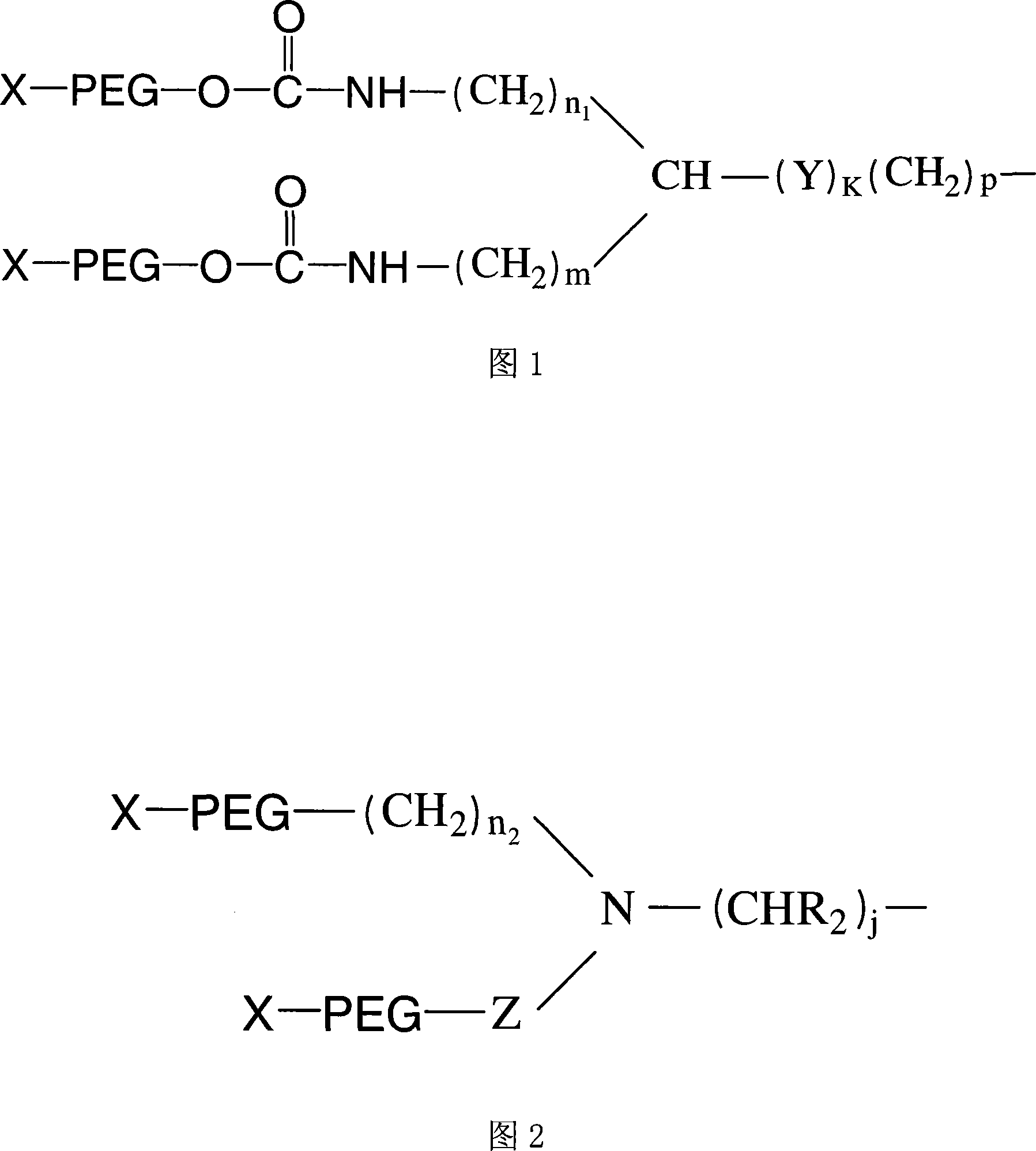Exendin or its analogs with polyethylene group and its preparation and application
A polyethylene glycol group, polyethylene glycol technology, applied in the field of modified Exendin or its analogues, can solve the problems of impractical application, increased dosage, and decreased biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The solid-phase chemical synthesis of embodiment 1 Exendin-4 analog
[0064] This embodiment introduces the synthesis of Exendin-4 analogs with the following structure by the method of solid-phase chemistry:
[0065] [His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Arg-Glu-Glu-Glu-Ala-Val-Lys-Leu-Phe-Ile-Glu-Trp -Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-OH] (SEQ ID NO: 232).
[0066] (1) Amino acid monomers used
[0067] Fmoc-L-Ala-OH
Fmoc-L-Lys(Boc)-OH
Fmoc-L-Asn(Trt)-OH
Fmoc-L-Met-OH
[0068] Fmoc-L-Asp(OtBu)-OH
Fmoc-L-Phe-OH
Fmoc-L-Gln(Trt)-OH
Fmoc-L-Pro-OH
Fmoc-L-Glu(OtBu)-OH
Fmoc-L-Ser(tBu)-OH
Fmoc-L-Gly-OH
Fmoc-L-Thr(tBu)-OH
Fmoc-L-His(Trt)-OH
Fmoc-L-Trp-OH
Fmoc-L-Ile-OH
Fmoc-L-Tyr(tBu)-OH
Fmoc-L-Leu-OH
Fmoc-L-Val-OH
[0069] The abbreviation in the above formula means:
[0070] Fmoc: 9-fluorenylmethoxycarbonyl
[0071] BOC: ...
Embodiment 2
[0093] The preparation of the genetic engineering method of embodiment two Exendin-4 analogs
[0094] This embodiment introduces the synthesis of the Exendin-4 analog with the following structure by the method of genetic engineering:
[0095] [His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Leu-Glu-Glu-Glu-Ala-Val-Lys-Leu-Phe-Ile-Glu-Trp -Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-Arg-OH]
[0096] (SEQ ID NO: 251).
[0097] A. According to the amino acid sequence of this Exendin-4 analog, synthesize the gene fragment:
[0098] (1) 5'AAT TCC ATG CAC GGC GAA ACC TTC ACC AGC GAT CTG AGC AAA CAG CTG GAA GAA GAAGCG GTT AA (SEQ ID NO: 266)
[0099] (2) 5' ACTG TTC ATC GAA TGG CTG AAA AAC GGC GGC CCG AGC AGC GGC CCG CCG CCG CCG AGCCGT TAG A (SEQ ID NO: 267)
[0100] (3) 5' AGCTT CTA ACG GCT CGG CGG CGG CGC GCT GCT CGG GCC GCC GTT TTT CAG CCA TTC GATGA (SEQ ID NO: 268)
[0101] (4) 5' ACAG TTT AAC CGC TTC TTC TTC CAG CTG TTT GCT CAG ATC GCT GGT GAA GGT GCC TTCGCC...
Embodiment 3
[0116] Example 3 Modification of Exendin-4 with linear monomethoxypolyethylene glycol (molecular weight=5,000 Daltons)
[0117] Weigh 1.0 mg Exendin-4 into each test tube, three test tubes in total. Dissolve in phosphate buffer solutions with different pH values, and then add 5.8 mg of N-hydroxysuccinimide-activated monomethoxypolyethylene glycol (molecular weight = 5,000 Daltons) to each test tube, place on a shaker, React at room temperature for 1 hour. Then use Agilent 1100 series chromatograph (Agilent 1100) to analyze samples (analytical conditions are: adopt 0.1% phosphoric acid mobile phase A and 0.1% phosphoric acid+80% acetonitrile mobile phase B, gradient is: 35-70% mobile phase B / 25 minutes ), to detect the amount of Exendin-4 not modified by monomethoxypolyethylene glycol. The results are as follows:
[0118] Table: Effects of different pH on PEGylation of Exendin-4
[0119] pH value
[0120] 8.5
[0121] Using a rotary evaporator to remov...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com