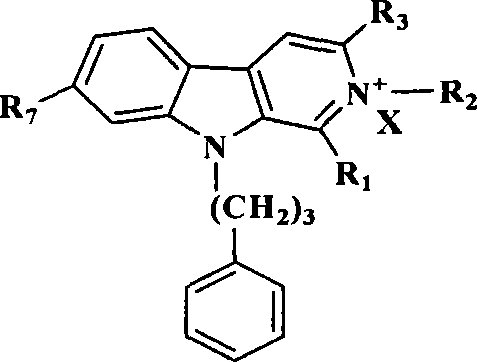
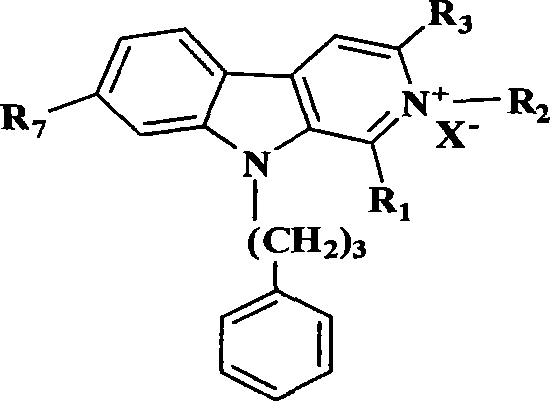
Banisterine derivative compound and uses thereof
A technology of dehydrohalamine and compounds, applied in the field of pharmaceutical compounds, can solve the problems of no clinical development and application, low anti-tumor activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
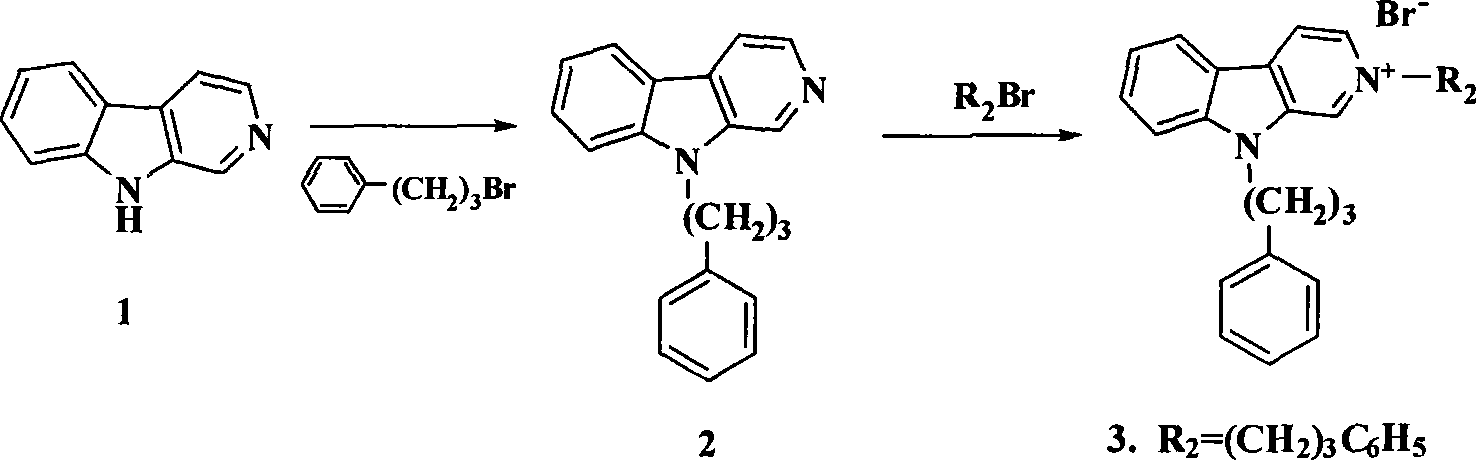
[0074] Example 1: Synthesis of 9-phenylpropyl-β-carboline (2)
[0075] Mix N,N-dimethylformamide (100ml), 1-bromo-3-phenylpropane (15mmol) and potassium iodide (15mmol), heat and stir in a water bath at 60°C for 3h, then cool the reaction solution to room temperature and add sodium hydride (1.2g, 30mmol) and β-carboline 1 (1.68g, 10mmol), the reaction was stirred at room temperature, followed by TLC detection. After the reaction was completed, the reaction mixture was poured into ice water, extracted with ethyl acetate, the organic phase was washed with water and saturated brine; then the organic phase was acidified with concentrated hydrochloric acid, and concentrated to dryness under reduced pressure. Anhydrous ethanol was taken with water several times, and the residue was used Acetone was recrystallized to obtain white crystals. Dissolve the white crystals in water, basify with sodium bicarbonate, extract with ethyl acetate, wash the organic phase with water, wash with saturat...
Embodiment 2
[0076] Example 2: General synthesis process of 2-substituted 9-phenylpropyl-β-carboline alkaloid derivatives (3-6)
[0077] 9-Phenylpropyl-β-carboline 2 (5mmol), add 75ml ethyl acetate to completely dissolve it, add the corresponding bromine or iodoalkane (10mmol), heat to reflux for 8 hours, cool to room temperature, filter the precipitated solid After washing with ethyl acetate, the solid was dissolved in 50ml of absolute ethanol, heated to reflux until clarified, filtered while hot, placed in the refrigerator to recrystallize, filtered, and washed with absolute ethanol to obtain the product.
[0078] Embodiments 3-6 are all operated according to the above operation steps:
Embodiment 32
[0079] Example 32, 9-diphenylpropyl-β-carboline bromide (3): yellow crystals were obtained, m.p. 120-121°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com