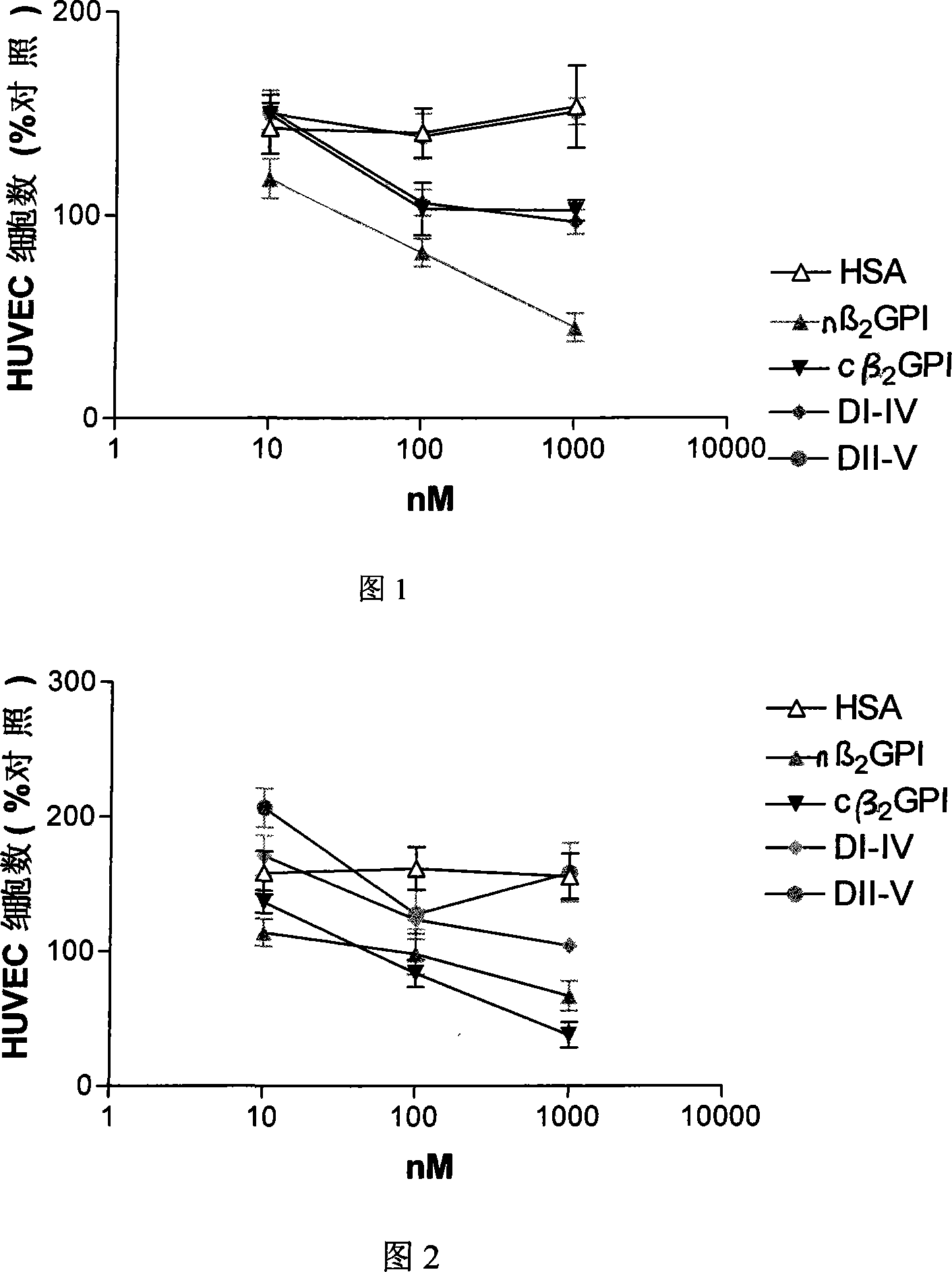
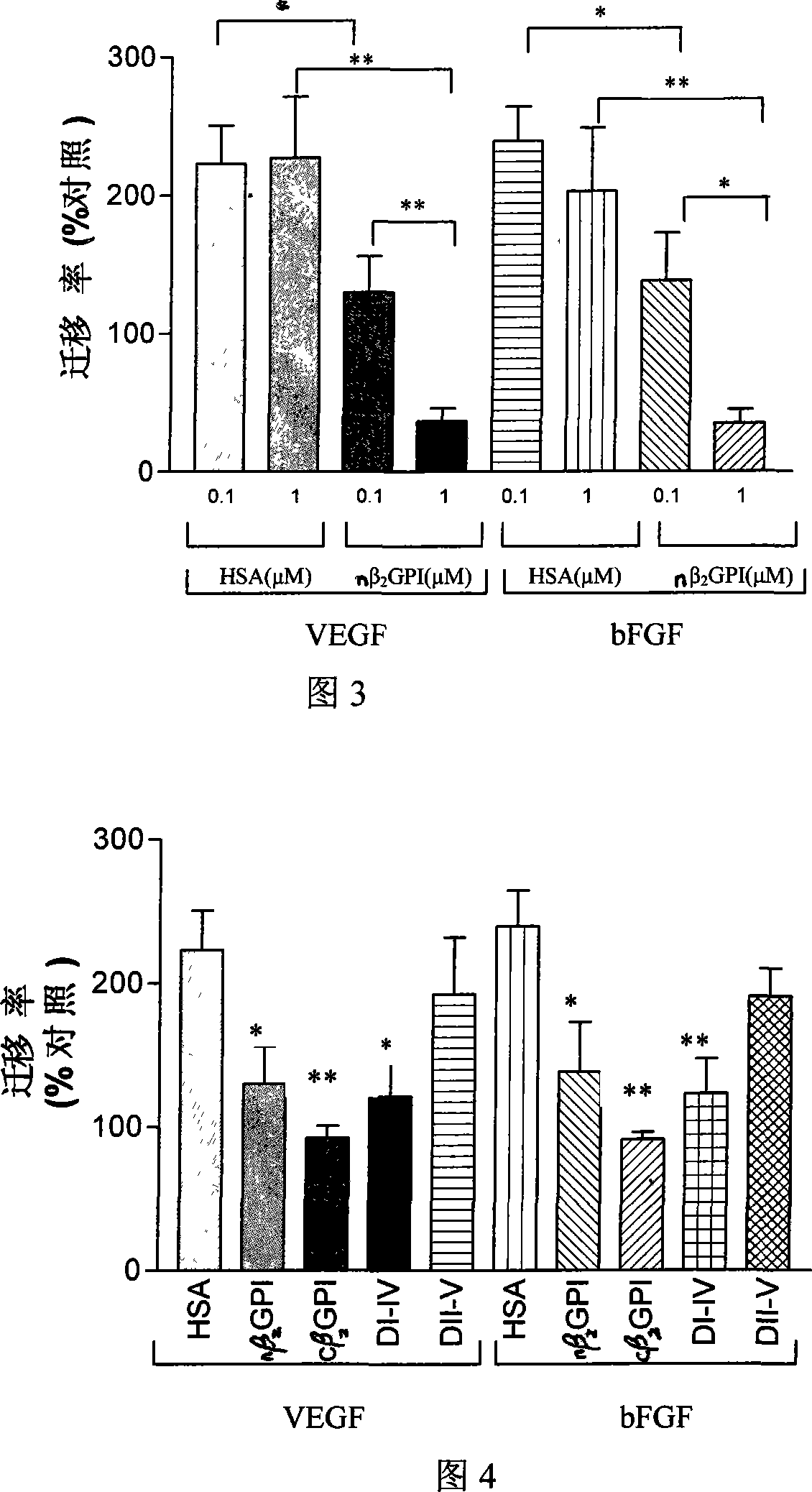
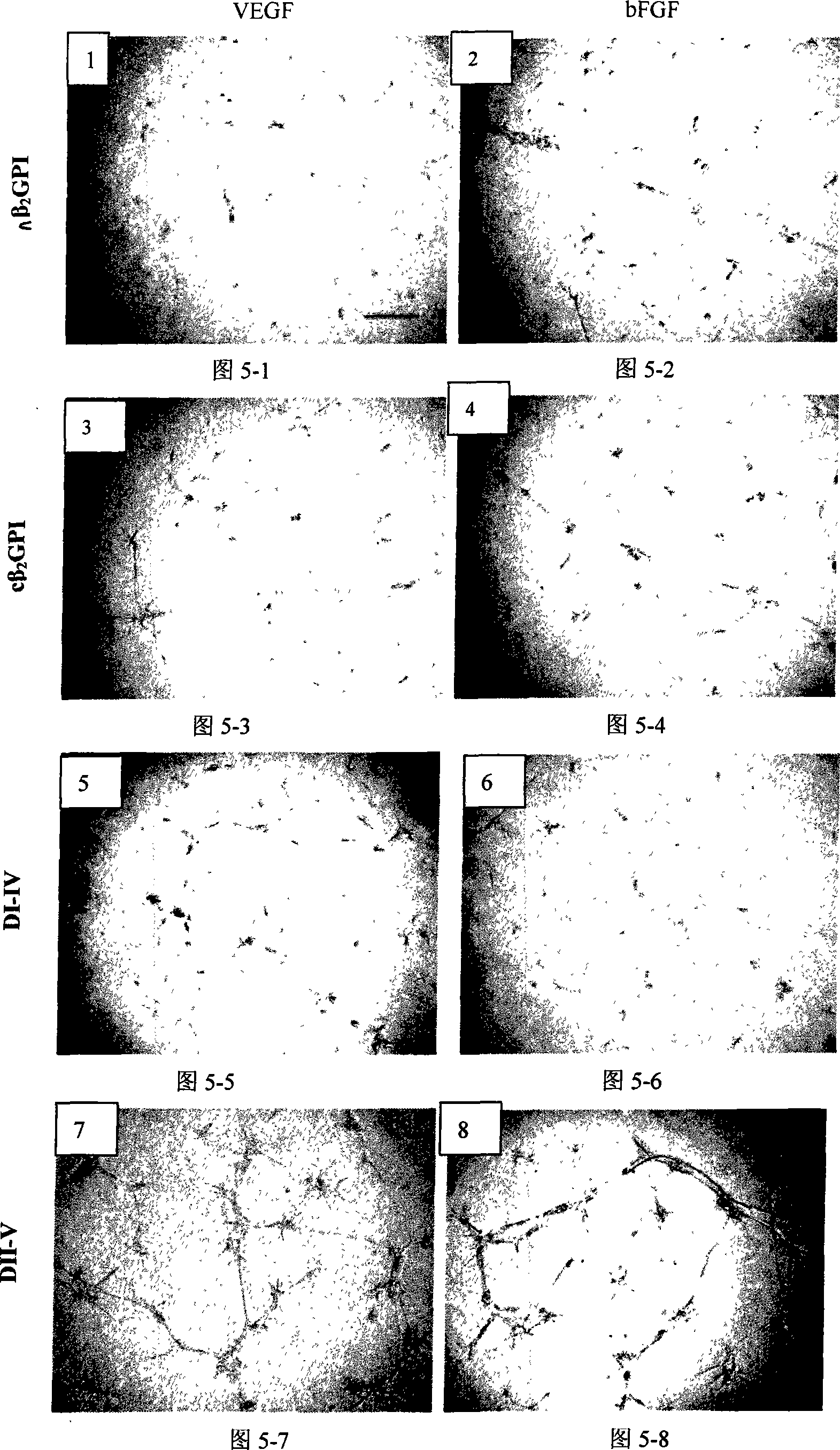
Application of natural type, breaking type or No. 5 domain delation variant type beta 2 glycoprotein I in preparing medicine for inhibiting blood vessel newborn
A technology of angiogenesis and deletion mutation, applied in drug combination, glycopeptide components, metabolic diseases, etc., can solve problems such as high cost, variable inhibition degree, complicated operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Materials and Methods
[0040] Reagent
[0041] For the amino acid sequences of native β2 glycoprotein I, V domain-deleted mutant β2 glycoprotein I (DI-IV) and I domain-deleted mutant β2 glycoprotein I (DII-V), see http: / / www.ncbi .nlm.nih.gov / entrez / viewer.fcgi? db=protein&id=28814 with serial number CAA40977.
[0042] Split β2 glycoprotein I (cβ 2 GPI) sequence see Hunt JE, Simpson RJ, Krilis SA. Identification of a region of beta2-glycoprotein I critical for lipid binding and anti-cardiolipin antibody cofactoractivity. Proc Natl Acad Sci U S A. 1993 Mar 15; 90 (6): 2141-5.
[0043] Plasma-derived native β2GPI (nβ 2 GPI) was purchased from Haematologic Technologies Inc (Essex Junction, VT).
[0044] Several preparation methods of β2 glycoprotein I:
[0045] (1) Expression and purification of V domain-deleted mutant β2 glycoprotein I (DI-IV) and I domain-deleted mutant β2 glycoprotein I (DII-V):
[0046] 1. Construction of a baculovirus vector containing a targe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com