Reversible over-charge protective electrolyte additive of lithium ion battery and its making method
A technology of electrolyte additive and lithium ion battery, applied in the field of electrolyte additive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
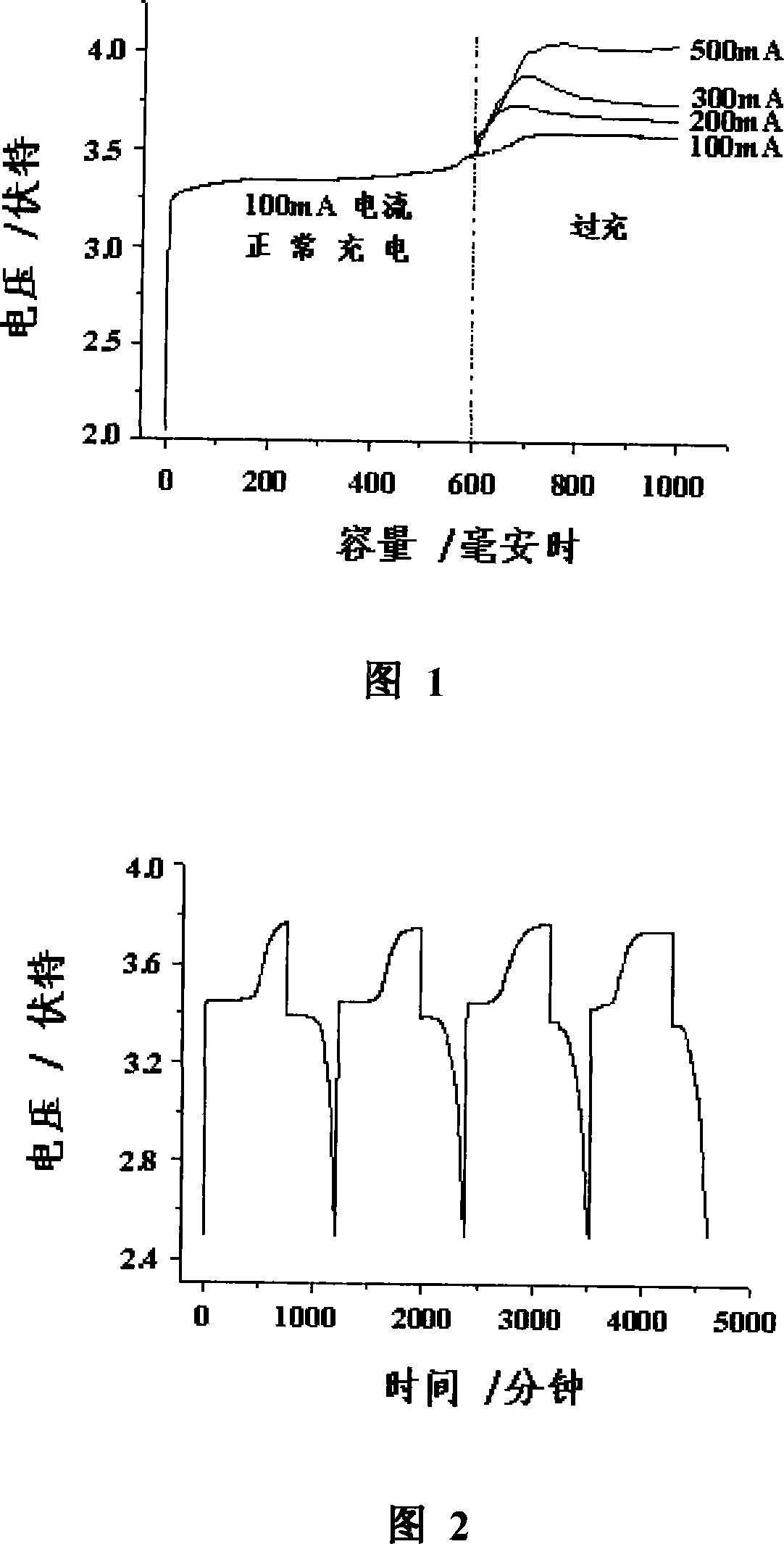
[0012] Diphenylamine was used as an electrolyte additive, and the battery used was a 103050 lithium-ion battery without liquid injection. Its cathode is LiFePO 4 , the negative electrode is graphite, and the electrolyte is 1mol / LLiPF dissolved with 5% (mass ratio) diphenylamine 6 / EC+DMC (volume ratio 1:1) solution.
[0013] After the battery was vacuum-dried at 85° C. for 24 hours, it was transferred to a dry glove box. Inject 3.5 ml of the above-mentioned electrolyte solution into the battery by means of vacuum pressure injection. After sealing, use 50mA current to form, and then repeat the electric overcharge test. The overcharge current is 100mA, 200mA, 300mA, 500mA respectively, and the overcharge degree is 100% of the battery capacity.
[0014] Experiments have found that when the battery is overcharged at the above-mentioned currents, there is a voltage plateau that does not rise with the increase of the overcharge degree. The voltage platforms at 100mA, 200mA, 300...
Embodiment 2
[0016] O-nitrodiphenylamine is used as the electrolyte additive, and the battery used is Li / LiFePO 4 Button batteries. Among them, the cathode is LiFePO 4 , the negative electrode is a lithium sheet, and the electrolyte is 1moi / L LiPF dissolved with 3% (mass ratio) o-nitrodiphenylamine 6 / EC+DMC (volume ratio 1:1) solution.
[0017] When the battery is charged and discharged at a constant current of 20mA / g, the charging voltage does not continue to rise with the progress of overcharging, but a stable voltage plateau appears at around 3.75V, which clearly shows the effect of additives on the overcharging voltage. Clamp function. After repeated charge and discharge cycles for 50 weeks, the battery overcharge platform has not changed significantly, and the battery capacity has remained basically stable. It shows that the additive can provide long-term and effective reversible overcharge protection for the battery.
Embodiment 3
[0019] Diphenylamine and triphenylamine were used as electrolyte additives, and the battery used was a 103050 lithium-ion battery without liquid injection. Its cathode is LiFePO 4 , the negative electrode is graphite, and the electrolyte is 1mol / L LiPF dissolved in 5% (mass ratio) diphenylamine and 2% (mass ratio) triphenylamine 6 / EC+DMC (volume ratio 1:1) solution.
[0020] After the battery was vacuum-dried at 85° C. for 24 hours, it was transferred to a dry glove box. Inject 3.5 ml of the above-mentioned electrolyte solution into the battery by means of vacuum pressure injection. After sealing, use 50mA current to form, and then repeat the electric overcharge test. Charge and discharge at a constant current of 500mA, and the degree of overcharge is 100% of the battery capacity. With the progress of overcharging, the charging voltage did not continue to rise, but a stable voltage plateau appeared at around 3.7V, which clearly showed the clamping effect of the additive o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com