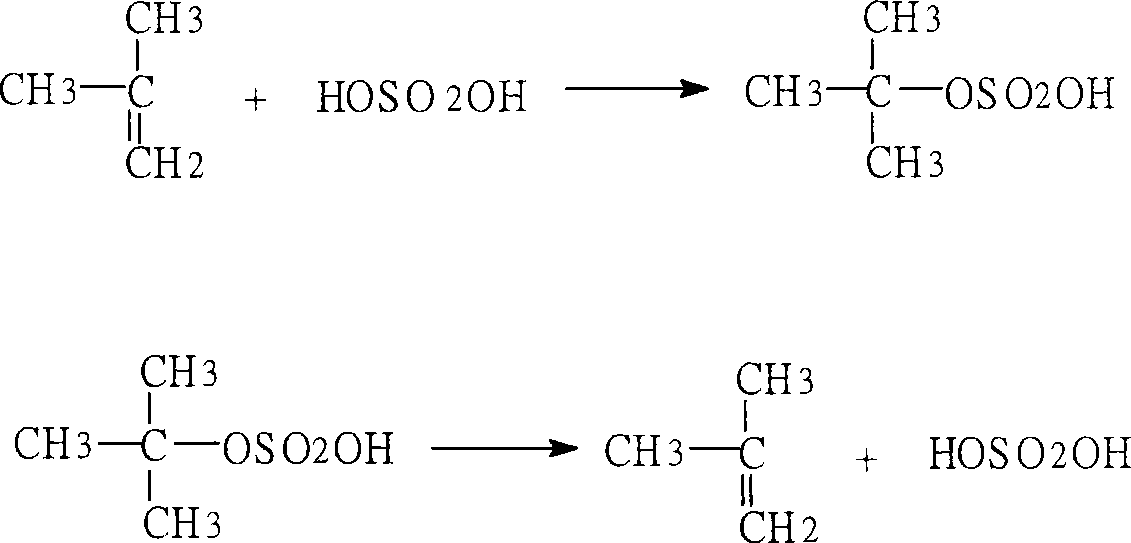
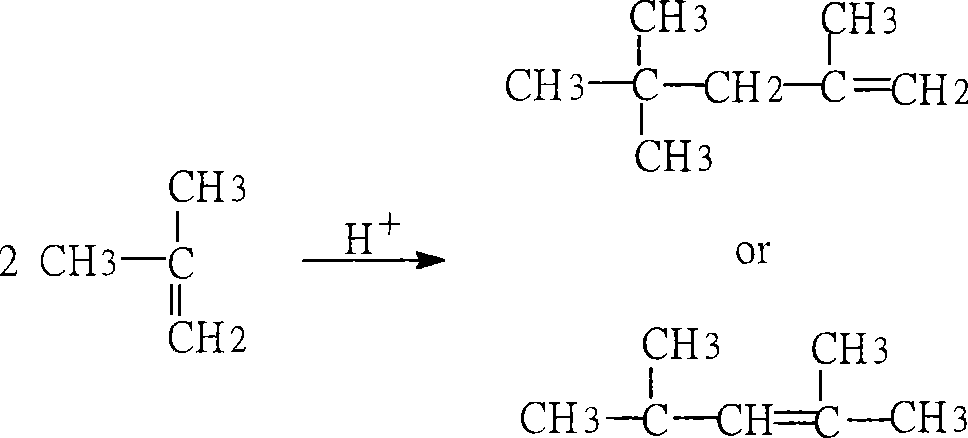
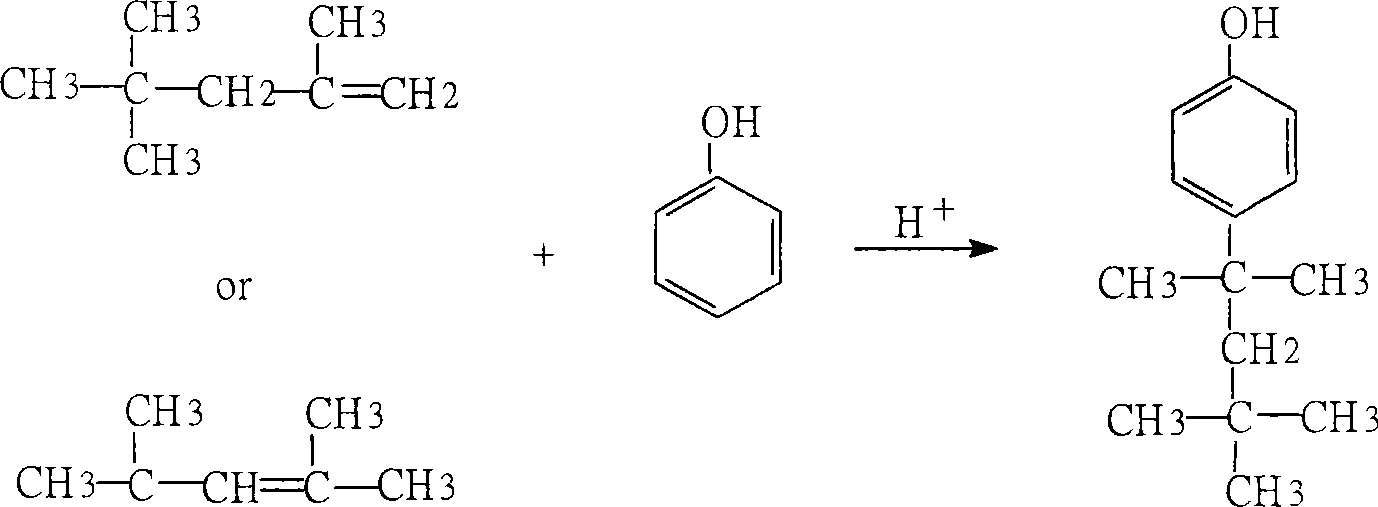
Method for preparing p-tert octyl phenol
A technology of tertoctylphenol and phenol, which is applied in the field of preparation of p-tertoctylphenol, achieves good application prospects, overcomes the difficulties of separation and treatment, and overcomes the effect of high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1, p-tertoctylphenol preparation method
[0020] Adopt the preparation method of p-tertoctylphenol of the present invention, with the content of about 80% C 4 The addition mixture (by-product) is used as the raw material; the isobutylene dimer with the content of the target fraction > 98% obtained by distillation and cutting at 75°C-105°C is used as an alkylating agent; Na-type sulfonated styrene-ethylene di Alkene copolymer strongly basic anion exchange resin, converted into H-type sulfonated styrene-ethylene-diene copolymer strongly acidic cation exchange resin after acidification, as an alkylation catalyst, undergoes alkylation reaction with phenol at 100°C , The alkylation mixture was cut by distillation at 220°C-270°C to obtain p-teroctylphenol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com