Reagent kit and method for detecting acute myocardial infarction variance biological mark
A technology for acute myocardial infarction and biomarkers, which is applied in the detection of biomarkers, biomarkers, non-invasive in vitro detection, and can solve the problem that disease markers cannot be detected at the same time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Discovery and distinction of protein fingerprint differences in blood between normal people and acute myocardial infarction
[0056] (1) Experimental method
[0057] 1. Materials
[0058] 1. Source of specimens: 100 patients in the acute myocardial infarction group, all diagnosed as acute myocardial infarction by electrocardiogram and myocardial enzyme spectrum; 100 normal people in the normal group. All three groups were between 45 and 60 years old and had similar exposure histories. The ratio of male to female is 1:1. None of the three groups had other related diseases affecting the protein content in serum.
[0059] 2. Reagents: urea, acetonitrile, trifluoroacetic acid, and SPA (Sinapinic acid) were all purchased from Sigma, and WCX matrix magnetic beads were from Celdy.
[0060] 2. Method
[0061] 1. Collection of samples: After the whole blood is collected, draw the serum and store it at -80°C; take out the serum sample in the refrigerator at -80°C, ...
Embodiment 2
[0074] Example 2 Sorting and Identification of Serum Biomarkers in Acute Myocardial Infarction
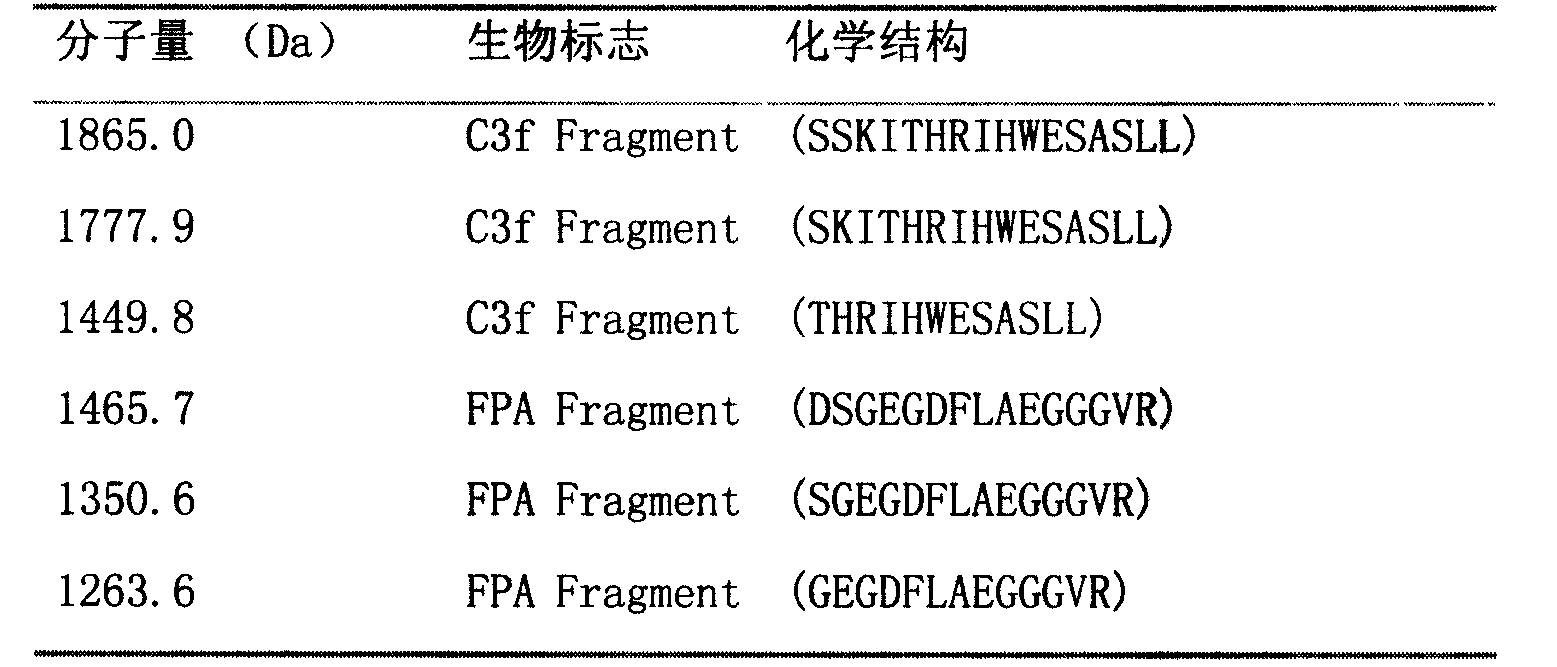
[0075] Select 1263.6±1Da, 1350.6±1Da, 1449.8±1Da, 1465.7±1Da, 1777.9±1Da, 1865.0±1Da biomarkers with MALDI-Qq-TOF multi-stage mass spectrometry (MS / MS), post-source fragmentation (PSD) and protein ladder Sort by protein ladder sequencing. By breaking molecules into pieces, protein ladders can be generated. This gradient is then analyzed by mass spectrometry. Biomarkers and their chemical structures were identified as variant complement C3f and variant fibrinopeptide A (arranged from N-terminus to C-terminus):
[0076] Table 1. Sequencing and identification of biomarker proteins in acute myocardial infarction
[0077]
[0078] FPA = fibrinopeptide A
[0079] The molecular weight of the complement C3f molecule in the known cDNA database is 2021.1Da, and the chemical structure is SSKITHRIHWESASLLR; the molecular weight of the fibrinopeptide A molecule in the known cDNA database...
Embodiment 3
[0080] Example 3 Molecular Identification of 42kDa Biomarker in Blood of Acute Myocardial Infarction
[0081] The Carbodiimide Method (Carbodiimide Method) was used to combine the matrix on the magnetic beads with carboxylic acid group labeling with the amino group of the anti-complement protein C3 alpha chain antibody (Gunn DL, et al.Preparation of sensitive and stable erythrocytes by the carbodiimide method for the detection of primary and secondary IgM and IgG antibody. J Immunol Methods. 1972; 1(4): 381-389.).
[0082] Samples were spotted on a matrix of anti-complement protein C3 alpha chain antibodies.
[0083] Wash with binding buffer. Apply the first wash solution to the site before the sample is completely dry. The wash solution was left on the spot for at least 10 seconds. Thoroughly remove the first wash solution and repeat the above steps with the second wash solution. Thoroughly wash the entire array point with 1% trifluoroacetic acid, elute the biomarkers ont...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com