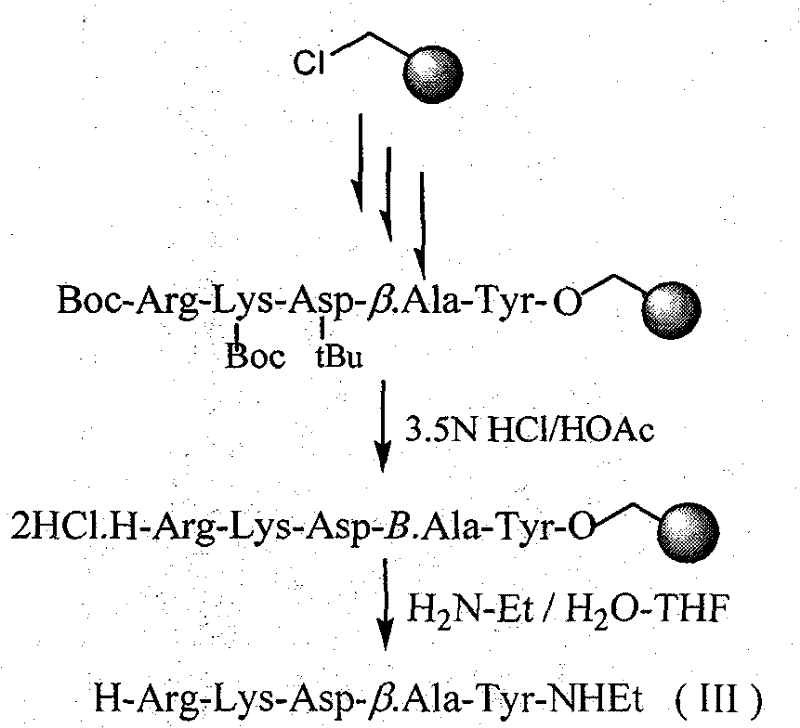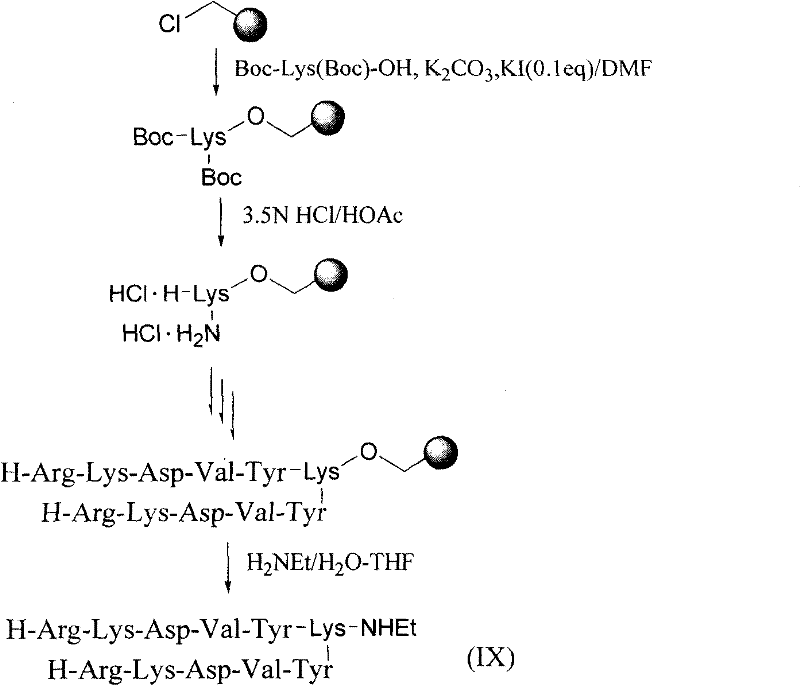N-substituted peptide amide, pharmaceutical composition and use thereof
A composition and drug technology, applied in the directions of drug combinations, peptides, cyclic peptide components, etc., to achieve the effects of strong biological stability and immunomodulatory activity, simple and feasible preparation method, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
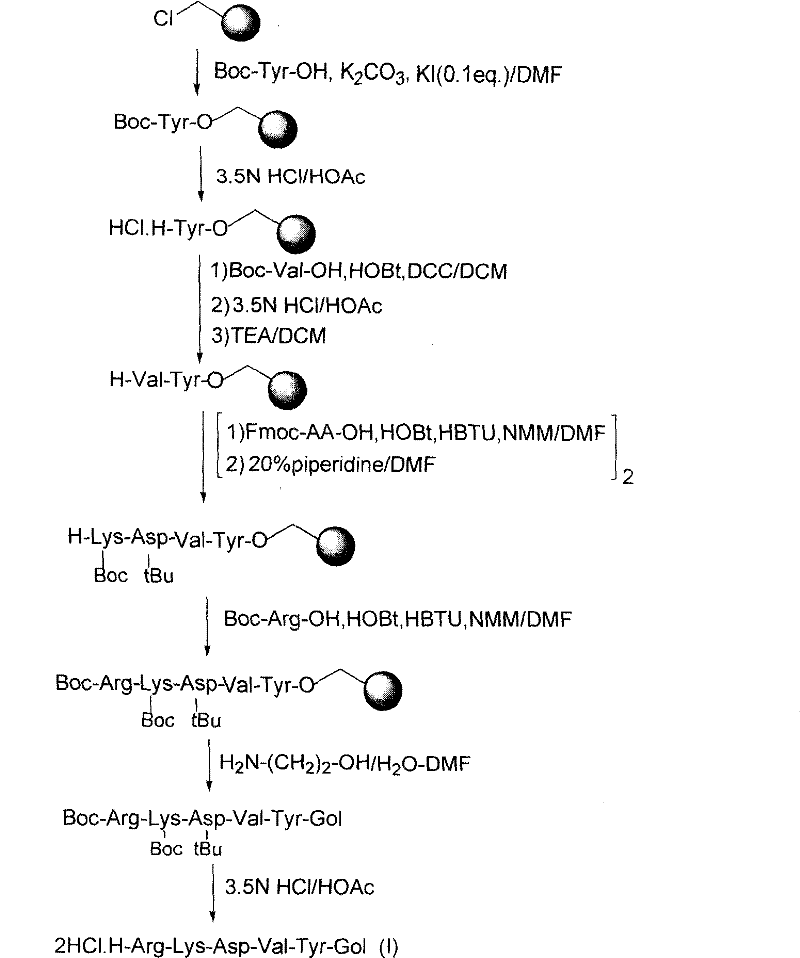
[0071] Synthesis of H-Arg-Lys-Asp-Val-Tyr-Gol·2HCl(I)
[0072] First, the classic solid-phase peptide synthesis strategy was adopted, using chloromethyl resin (substitution degree: 1 mmol / g, cross-linking degree: 1%, particle size: 100-200 mesh) as the solid-phase carrier, and the synthesis of the target sequence was completed step by step. Assemble. Among them, Tyr, Val and Arg all use Boc to protect the a-amino group, and the protection forms of the other two amino acids are: Fmoc-Lys(Boc)-OH and Fmoc-Asp(OtBu)-OH. The degree of condensation was monitored by ninhydrin color reaction after each condensation step. After all the condensation is completed, use aminoethanol (Gol) / H 2 O / DMF (2:1:3, v / v) solution was mixed with fully protected peptide resin and shaken for 30 h. The collected filtrate was mixed with an appropriate volume of distilled water, then passed through the C-18 filter layer under reduced pressure, and then rinsed with an appropriate volume of distilled wa...
Embodiment 2
[0078] H-Arg-Lys-Asp-Val-Tyr-NH(CH 2 ) 2 NH(CH 2 ) 2 The solid-phase synthesis of the synthetic peptide sequence of OH 2HCl(II) is the same as in Example I, except that the ammonolysis uses H 2 N(CH 2 ) 2 NH(CH 2 ) 2 OH is an amine component.
[0079] Other concrete reaction conditions are also the same with embodiment 1.
[0080] The yield of II was 87.5%,
[0081] Its ESI-MS analysis result was 766.3 (M+H).
Embodiment 3
[0083] Synthesis of H-Arg-Lys-Asp-βAla-Tyr-NHEt(III)
[0084] In the solid-phase synthesis of the peptide chain sequence, Boc-β-Ala-OH was used to replace the original Boc-Val-OH at the second position of the C-terminus, and the rest of the conditions were the same as in Example 1. In addition, another difference from Example I is that the Boc and tBu protecting groups are removed before the ammonolysis, and the free product IIIi is obtained after the ammonolysis:
[0085]
[0086] The yield of III is 93.6%, and the result of ESI-MS is: 679.4 (M+H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com