Uses of modified chitosan, and nanometer complex containing modified chitosan
A nano-composite, chitosan technology, applied in the field of medicine, can solve the problems of difficulty in permeation, the influence of insulin stability, and the improvement of oral absorption of incapable protein polypeptide drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0083] Preparation of Example 1 Modified Chitosan
[0084] Take raw material chitosan (Mw is 100,000, dynamic viscosity is 65mpa s, Zhejiang gold shell, degree of deacetylation is 90%) 3g, add 1% (v / v) acetic acid aqueous solution and stir at room temperature, then add sodium deoxycholate (900mg) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) (528mg), adjust the pH to 5.5 with aqueous sodium hydroxide solution, after 24 hours of reaction, use hydrogen oxidation Adjust the pH to 9.0 with sodium aqueous solution, centrifuge the precipitate, dialyze with a dialysis bag (MWCO 50,000), freeze-dry, and obtain 2.5 g of yellow floc after drying, which is modified chitosan.
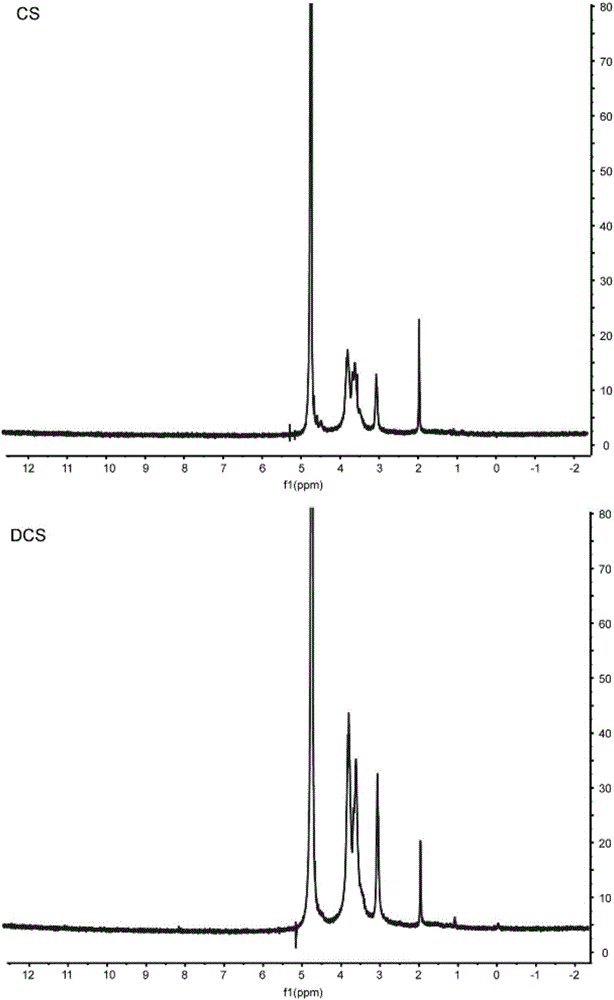
[0085] The prepared modified chitosan and the raw material chitosan were dissolved in deuterated acetic acid and then analyzed by proton nuclear magnetic resonance (Varian-MERCURY Plus-300, USA).
[0086] Compared figure 1 The NMR spectra of chitosan (CS) and deoxycholic acid-coupled chitosa...
preparation Embodiment 2
[0091] The preparation of preparation example 2 modified chitosan
[0092] Get raw material chitosan (Mw is 200,000, dynamic viscosity is 140mpa s, Zhejiang gold shell, deacetylation degree is 85%) 3g add 1% (v / v) acetic acid aqueous solution and stir at room temperature, add sodium deoxycholate successively (1800mg) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) (1056mg), adjust the pH to 5.5 with aqueous sodium hydroxide solution, after 24 hours of reaction, use hydrogen oxidation Adjust the pH to 9.0 with sodium aqueous solution, centrifuge the precipitate, dialyze with a dialysis bag (MWCO 100,000), freeze-dry, and obtain 2.5 g of yellow floc after drying, which is modified chitosan.
[0093] The degree of substitution of deoxycholic acid was measured with an elemental analyzer to be about 3.2%.
preparation Embodiment 3
[0094] Preparation of Example 3 Modified Chitosan
[0095] Get raw material chitosan (Mw is 100,000, dynamic viscosity is 65mpa s, Zhejiang gold shell, deacetylation degree is 90%) 3g adds 1% (v / v) acetic acid aqueous solution and stirs at room temperature, adds taurocholic acid successively Sodium (900mg) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) (528mg), adjust the pH to 5.5 with aqueous sodium hydroxide solution, after 24 hours of reaction, use hydrogen Adjust the pH to 9.0 with sodium oxide aqueous solution, centrifuge the precipitate, dialyze with a dialysis bag (MWCO 50,000), freeze-dry, and obtain 2.4 g of yellow floc after drying, which is modified chitosan.
[0096] The degree of substitution of sodium taurocholate was measured by an elemental analyzer to be about 1.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com