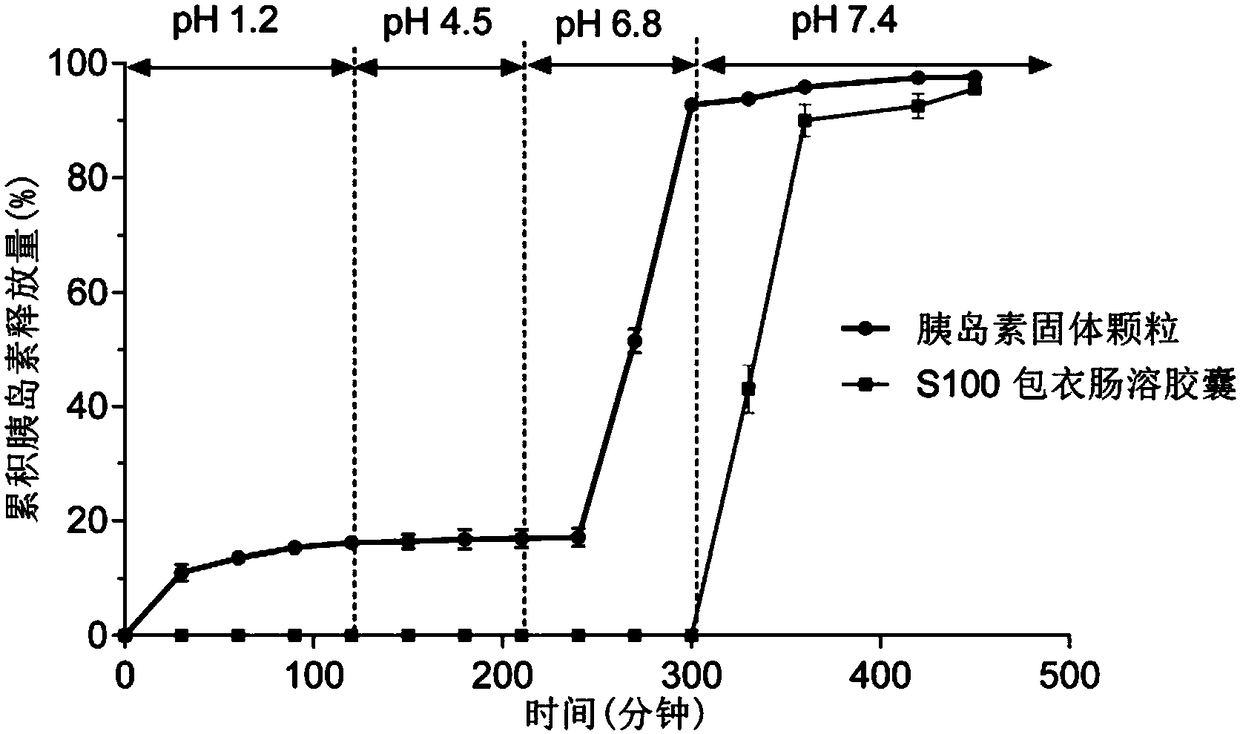
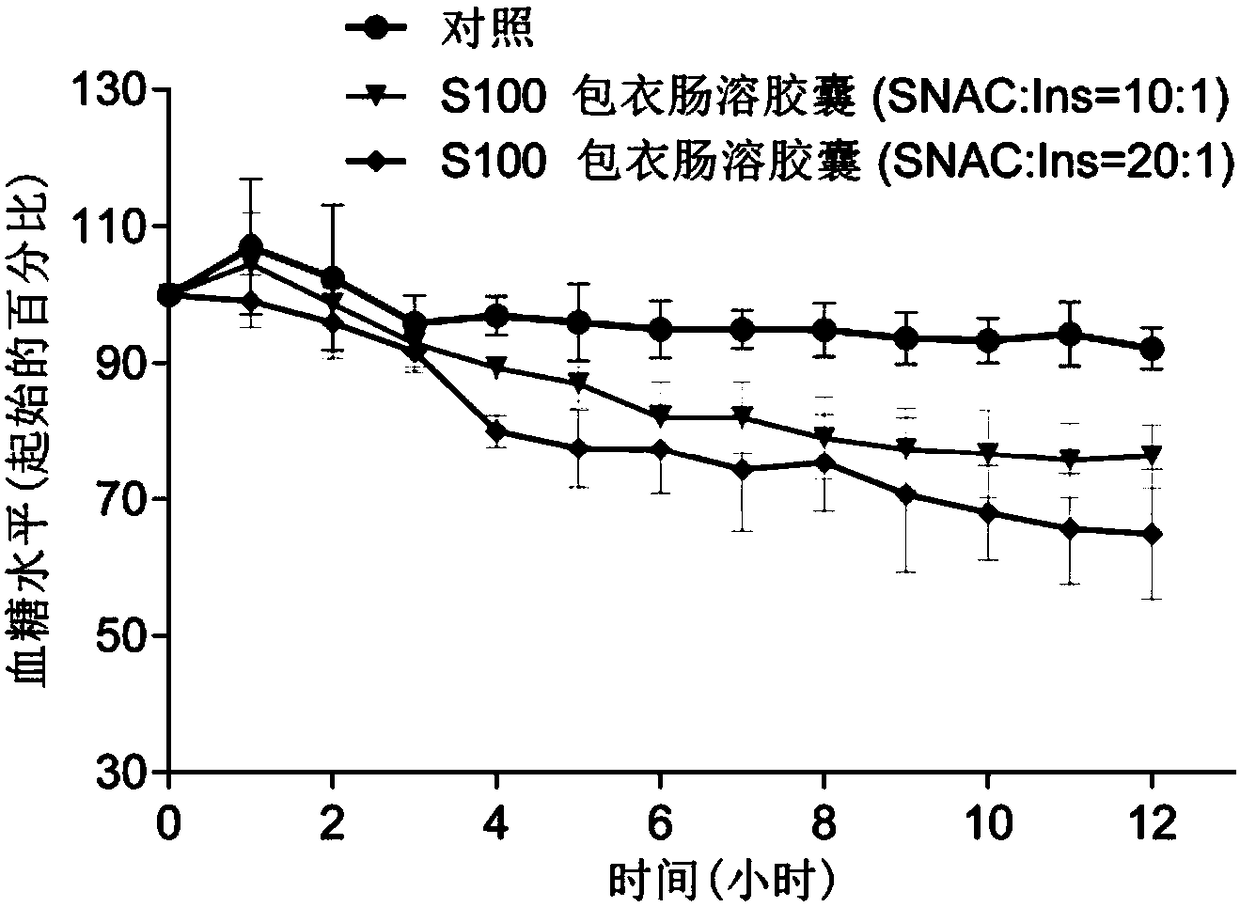
Polypeptide-protein-drug-carried solid particulate matter with mucous penetrability and preparation containing same, and preparing methods and application of polypeptide-protein-drug-carried solid particulate matter with mucous penetrability and preparation
A solid particle, protease inhibitor technology, applied in peptide/protein components, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of complex preparation process, difficulty, cell damage, etc. The preparation process is simple, the cost is low, and the effect of promoting absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0062] Preparation Example 1 Preparation of Insulin Granules Encapsulated by Pluronic F127 / PLGA
[0063] Dissolve 10mg of SNAC in 0.5mL of 0.01M NaOH aqueous solution, and dissolve 1mg of insulin (Ins) and 1mg of aprotinin in 0.5mL of 0.01M NaOH aqueous solution (containing 1% Tween 20) and add them to the SNAC solution to form a uniform Solution (A); 80mg Pluronic F127 / and 10mg PLGA were added to 4mL of ethanol / dichloromethane (ethanol:dichloromethane=1:4), which was fully dissolved to form a homogeneous solution (B); slowly added A to In B, after vortex mixing at 500r / min to form W / O emulsion (C), slowly add (C) dropwise into 20mL liquid paraffin (containing 1% Span 80) to form W / O / O microspheres; add The microspheres were solidified with n-hexane, continued to stir for 1.5 h, washed with n-hexane for 3 times, put the microspheres into a vacuum drying oven to dry for 24 h, and obtained Pluronic F127 / PLGA-coated insulin granules.
preparation Embodiment 2
[0064] Preparation Example 2 Preparation of Insulin Granules Encapsulated by Pluronic F127 / Eudragit S100
[0065] Dissolve 100mg of SNAC in 1mL of 0.01M NaOH aqueous solution, and dissolve 10mg of Ins and 10mg of aprotinin in 1mL of 0.01M NaOH aqueous solution, and add them to the SNAC solution to form a homogeneous solution (A); mix 200mg of Pluronic F127 and 200mg of Eudragit S100 Add to 48mL 95% ethanol (containing 10mL H 2 O) fully dissolved to form a solution (B); slowly add A to B, and vortex mix at 500r / min to form a homogeneous solution, and then use spray drying technology to prepare insulin granules wrapped in Pluronic F127 / Eudragit S100.
preparation Embodiment 3
[0066] Preparation Example 3 Preparation of Insulin Granules Encapsulated by Pluronic F127 / Eudargit S100
[0067] Dissolve 200mg of SNAC in 1mL of 0.01M NaOH aqueous solution, and dissolve 10mg of Ins and 10mg of aprotinin in 1mL of 0.01M NaOH aqueous solution, and add them to the SNAC solution to form a homogeneous solution (A); mix 200mg of Pluronic F127 and 200mg of Eudragit S100 Add to 48mL 95% ethanol (containing 10mL H 2 O) fully dissolved to form a solution (B); slowly add A to B, and vortex mix at 500r / min to form a homogeneous solution, and then use spray drying technology to prepare insulin granules wrapped in Pluronic F127 / Eudragit S100.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com