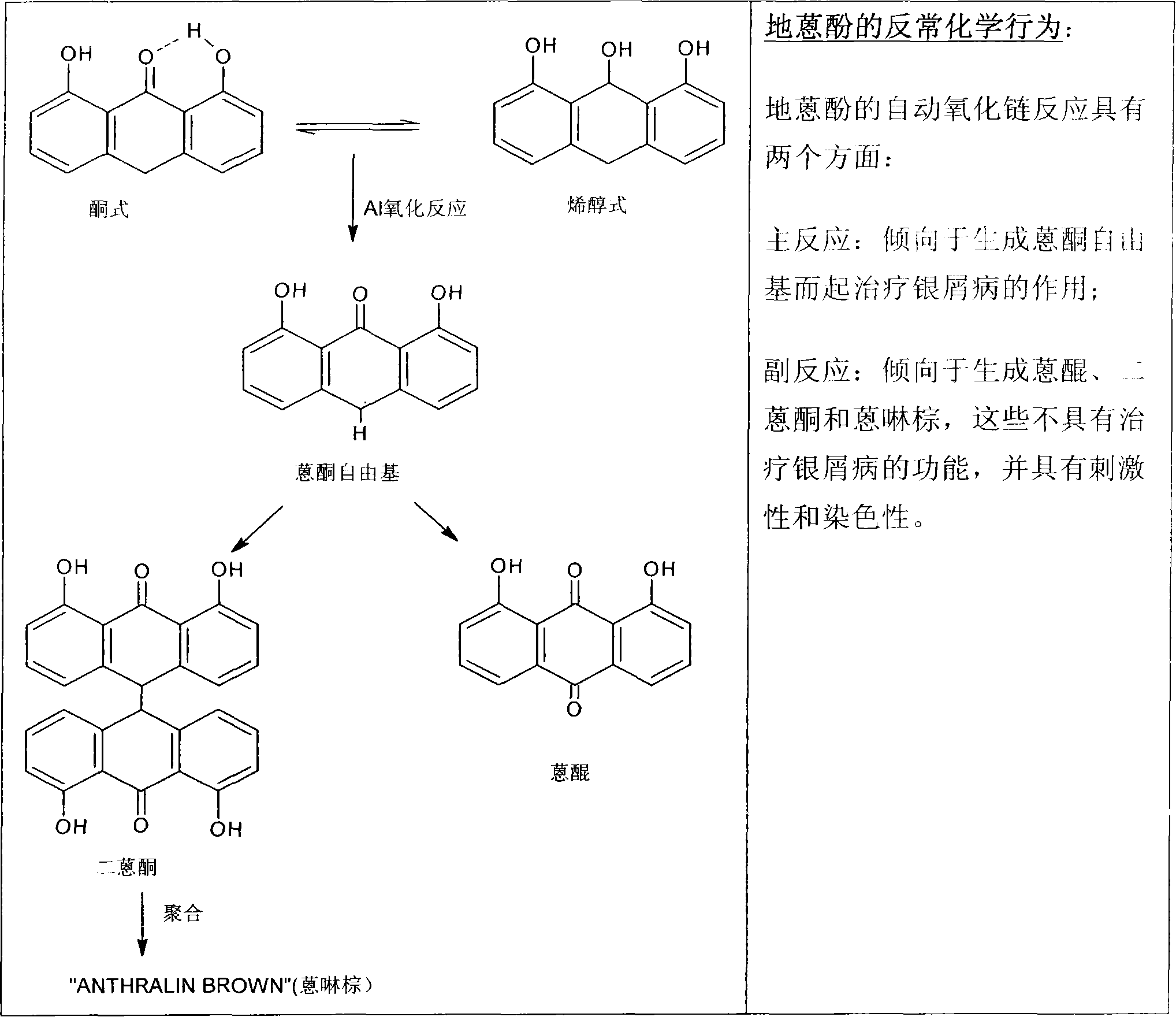
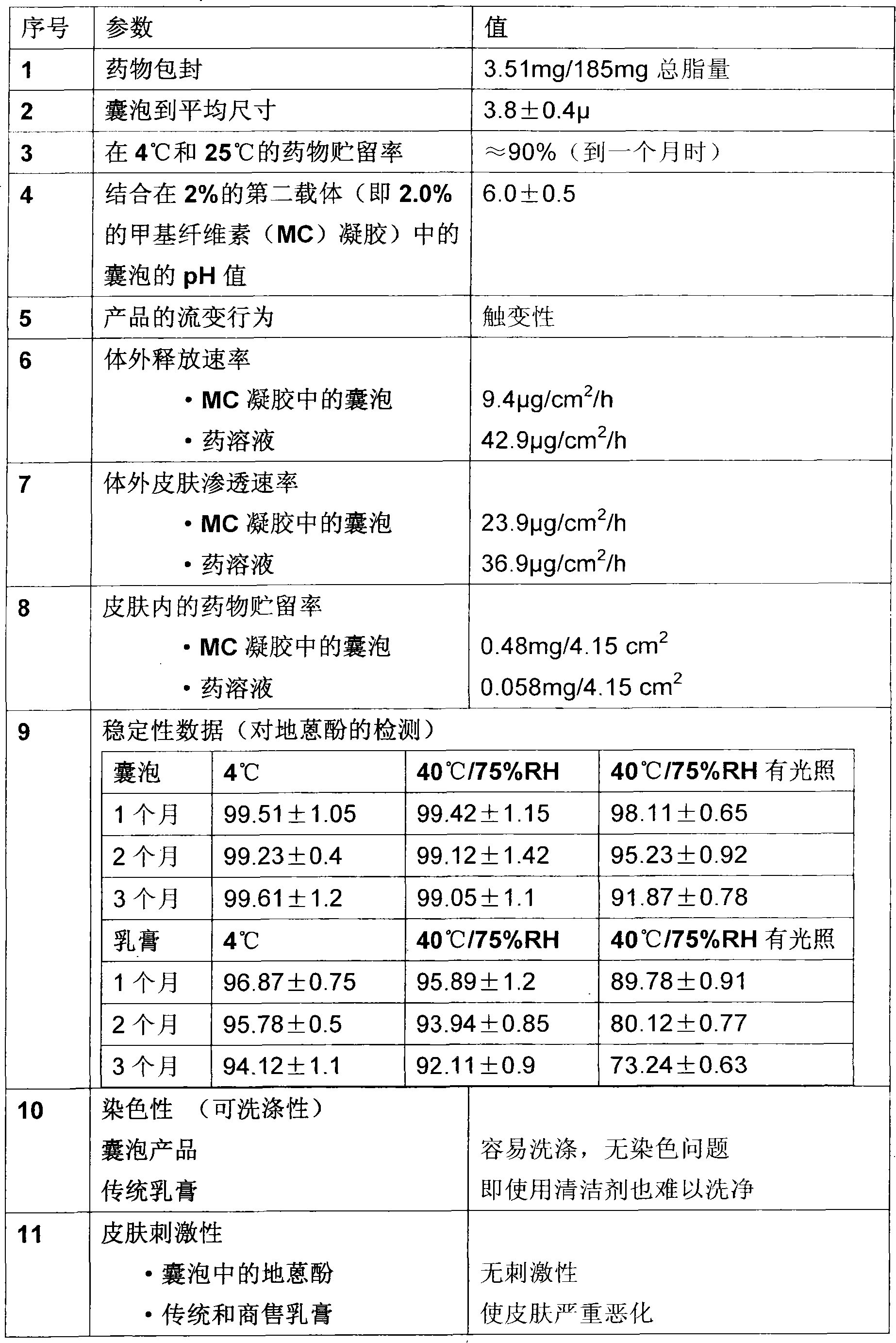
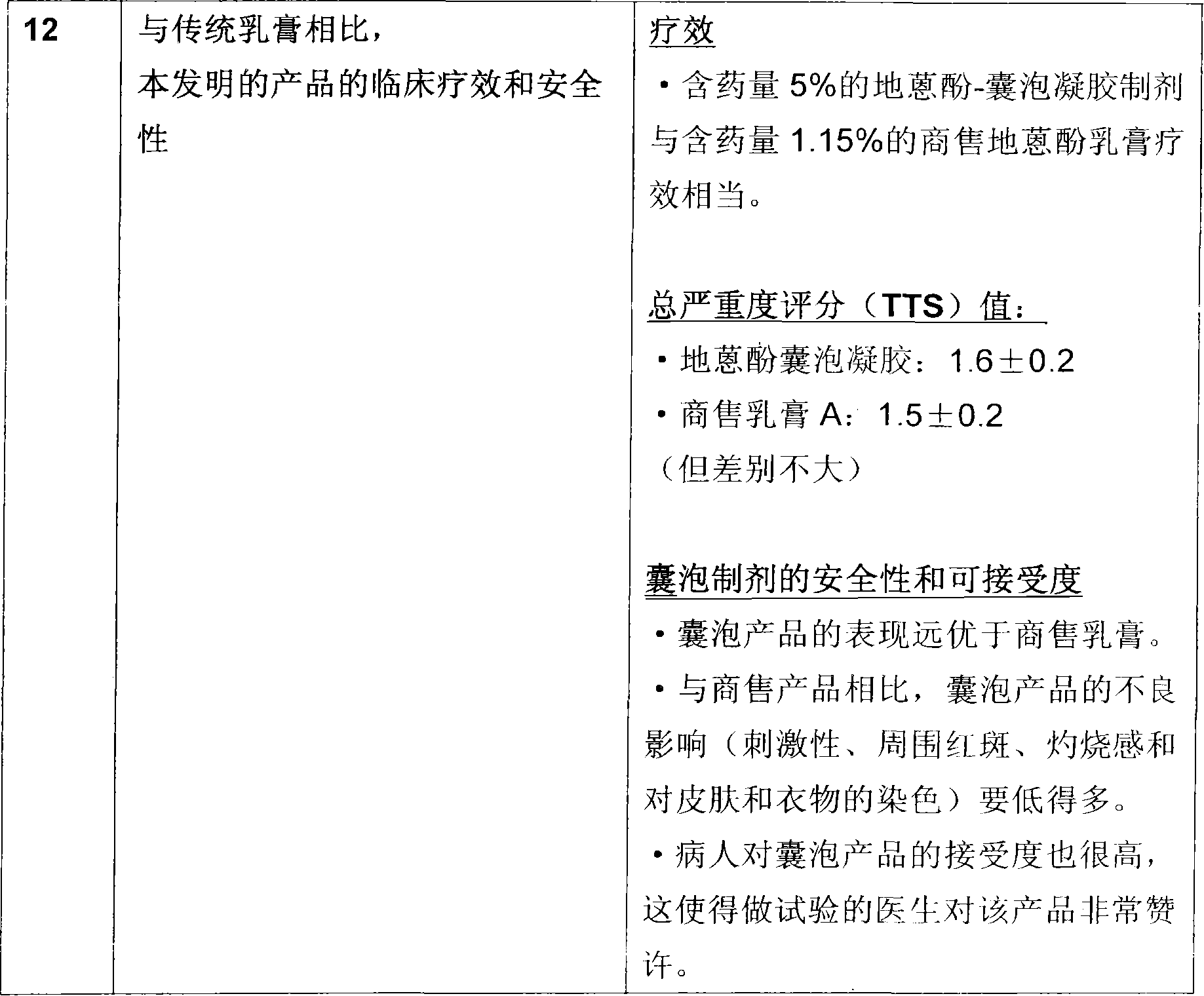
A novel inter and intra multilamellar vesicular composition
A technology for multi-layer vesicles and products, applied in the field of drug delivery, can solve the problems of low staining and low dispersion, and achieve the effect of improving patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0107] The composition of the obtained dithranol liposome product
[0108] serial number
component name
In every 20gm product
Amount of ingredients contained
%w / w
1
Dithranol
100mg
0.5
2
Phospholipids
2.0gm
10.0
3
1.0gm
5.0
4
Butylated Hydroxytoluene (Antioxidant)
60.0mg
0.3
5
Methylcellulose
400mg
1.0-2.0
6
USP Pharmaceutical Grade Phosphate Buffered Saline at pH 6.4
Contains 0.5% w / w sodium metabisulfite (antioxidant)
q.s.20.0gm
(q.s.: sufficient quantity)
[0109] parameter
value
Dithranol per Whole Product
0.5%w / w
Dithranol: total lipid (weight ratio)
100mg drug: 3000mg total fat
Dithranol as a percentage of total lipids
3.33% of total fat
intralaminar (intravesicular) dithranol
70.2% of the total dosage
interlaminar (int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com