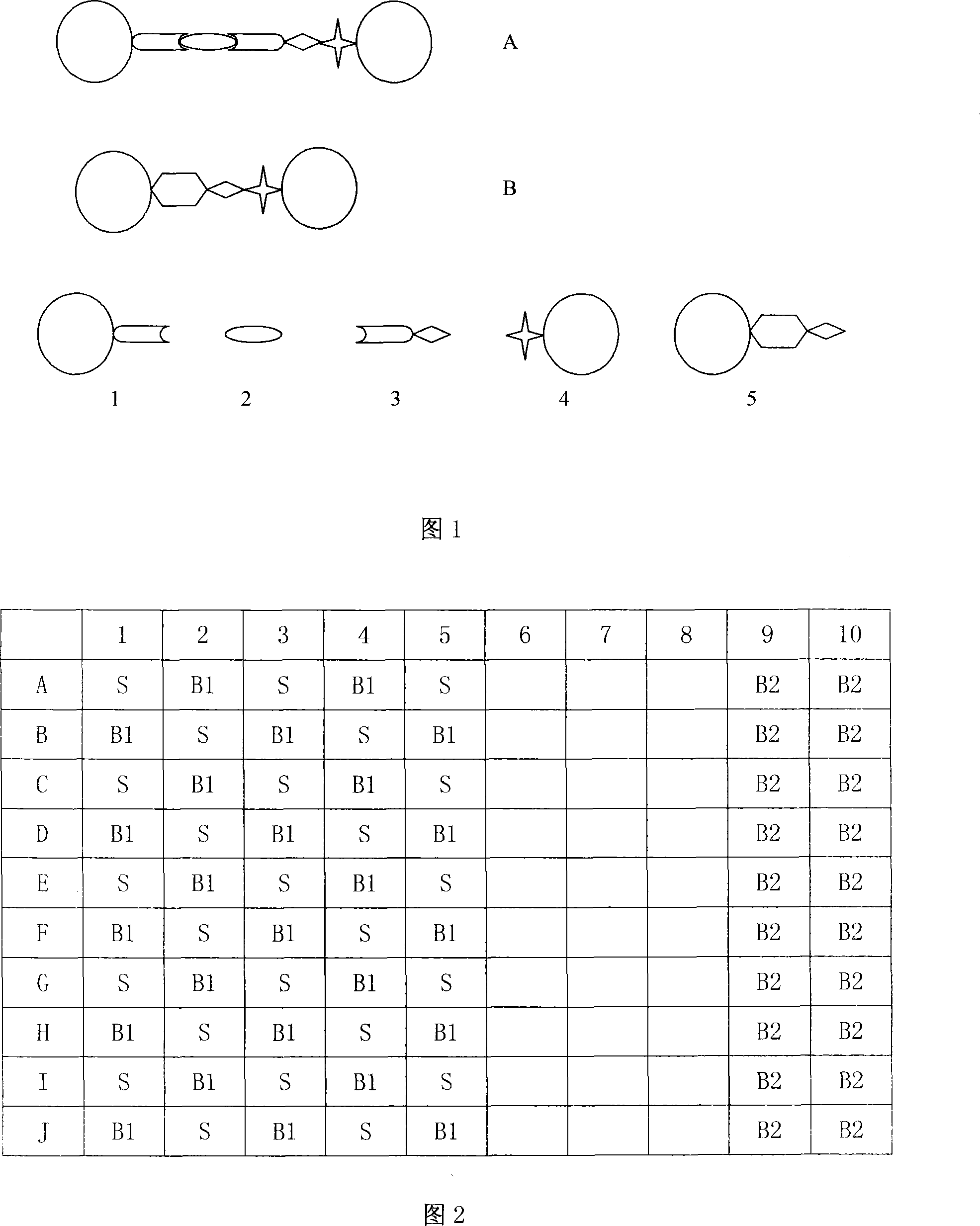
Quality controlled microparticles, preparation thereof and uses thereof
A microparticle and quality control technology, which is applied in the field of quality control reagents of instruments, can solve the problems of unfavorable long-term stable observation and large batch-to-batch differences, and achieve the effects of facilitating inter-laboratory quality evaluation, easy control, and reducing sample detection differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Preparation of quality control microparticles coated with biotin-labeled γ-globulin (BGG):
[0042] (1) Preparation of biotin-labeled BGG
[0043] Use O.1M NaCNBH 3 Prepare BGG (purchased from Pel-Freez Biological) into a 10mg / ml solution, and use DMSO (dimethylformamide) to configure Biotin-XX-NHS (N-hydroxysuccinimide modified biotin, manufacturer: SIGMA, product No.: B3295) solution to 16.172mg / ml, add 5.4ul Biotin-XX-NHS according to the amount of 1mg antibody to add Biotin-XX-NHS solution to the BGG solution, mix well and place it at 4°C overnight. Use dialysis to remove free unreacted biotin. The dialysate is biotin-labeled antibody dialysis buffer (0.1M Tris, 0.3M NaCl, 0.005M EDTA-Na-2H) 2 O, pH 8.0). After the dialysis, the protein concentration was determined by the BCA method.
[0044] (2) Adopt the above-mentioned labeled protein to coat aldehyde-modified luminescent particles
[0045] Add 6.4μl of 20% Tween-20 solution, 2mg of the biotin-labeled prot...
Embodiment 2
[0049] Example 2: Preparation of quality control microparticles coated with biotin-labeled bovine serum albumin (BSA)
[0050] (1) Preparation of biotin-labeled bovine serum albumin (BSA)
[0051] Use 0.1M NaCNBH 3 Prepare BSA (purchased from EQUITECH-BIO INC) into a 10mg / ml solution, and use DMSO (dimethylformamide) to configure Biotin-XX-NHS (N-hydroxysuccinimide modified biotin, manufacturer: SIGMA, product No.: B3295) solution to 16.172mg / ml, add 13.5ul Biotin-XX-NHS according to the amount of 1mg antibody to add Biotin-XX-NHS solution to the BSA solution, mix well and place at 4°C overnight. Use dialysis to remove free unreacted biotin. The dialysate is biotin-labeled antibody dialysis buffer (0.1M Tris, 0.3M NaCl, 0.005M EDTA-Na-2H) 2 O, pH 8.0). After the dialysis, the protein concentration was determined by the BCA method.
[0052] (2) Adopt the above-mentioned labeled protein to coat aldehyde-modified luminescent particles
[0053] The coating method is the same as in Exa...
Embodiment 3
[0057] Example 3: The maximum count rate of a quality control particle detection instrument
[0058] Add different concentrations of quality control particles to the instrument, and each concentration can be repeatedly measured. The concentration of quality control particles gradually increases. Then add anti-biotin-coated photosensitive particles to the above-mentioned wells, incubate for 15 minutes, and read per unit time. When the concentration of quality control particles increases, the signal value no longer rises or the signal overflow occurs, the highest signal value divided by the unit time is the maximum counting rate of the instrument.
[0059] Using the above method, we used the biotin-labeled BGG-coated quality control particles in Example 1 to detect the maximum count rate of the LiCA HT photoinduced chemiluminescence system (Shanghai Boyang Medical Instrument Co., Ltd.). The concentration of the quality control particles were 20, 40, 60, 80μg / ml, add 30μl to each wel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com