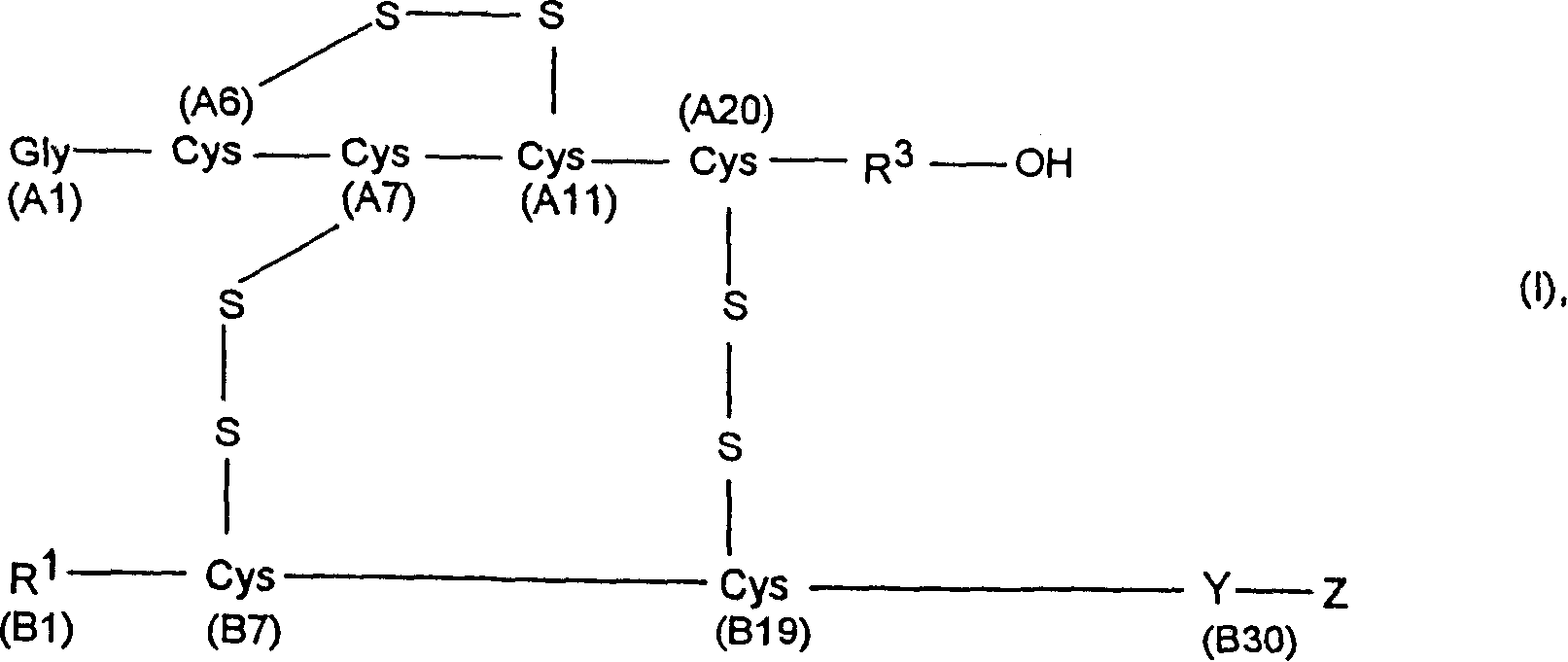
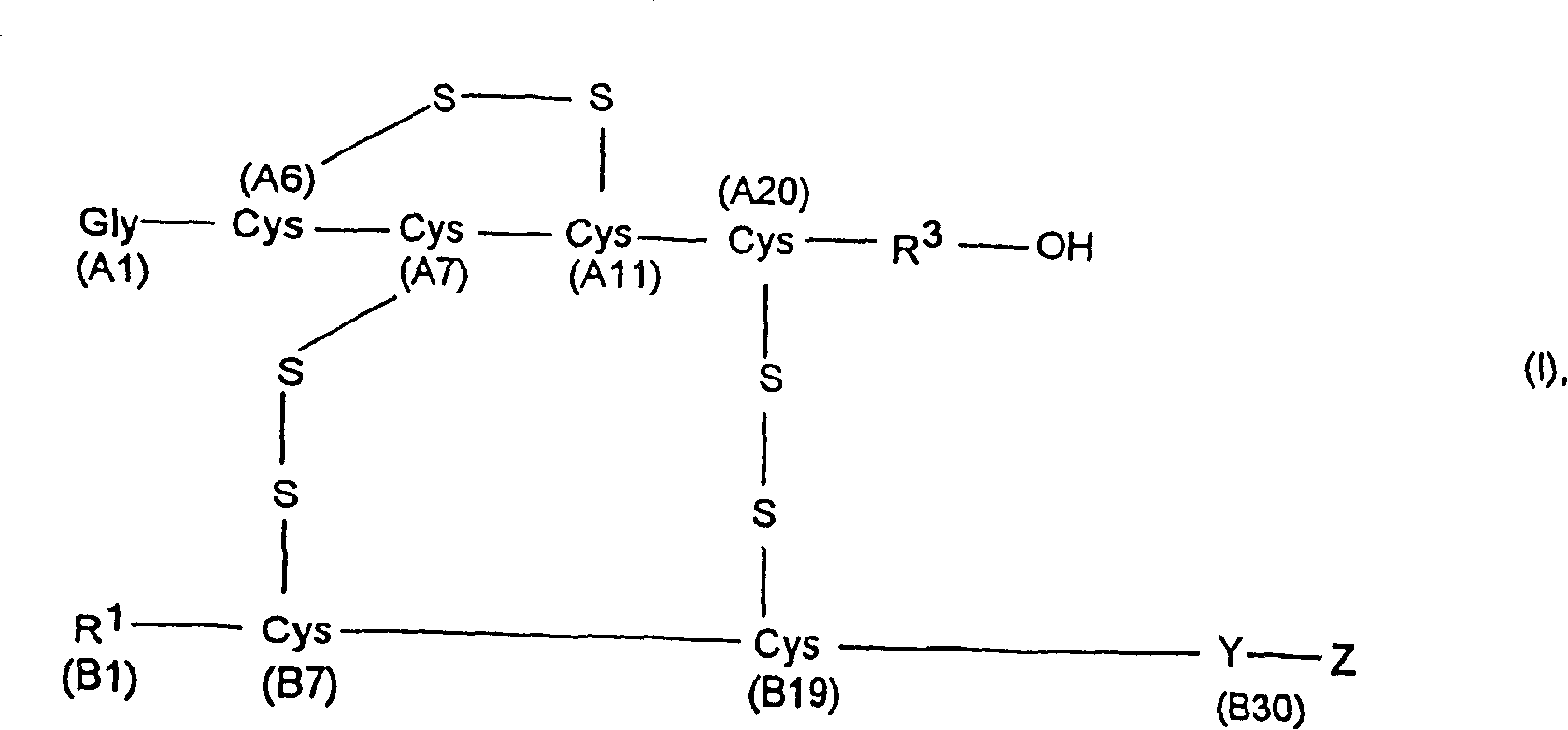
Process for obtaining insulin or insulin derivatives having correctly bonded cystine bridges
A technology of insulin derivatives and insulin, applied in the direction of microbial-based methods, biochemical equipment and methods, insulin, etc., capable of solving problems such as loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1 (comparative example, prior art)
[0096] The genetically modified E. coli cells were fermented (EP 0489780) to prepare a fusion protein with the following amino acid sequence.
[0097] Proinsulin sequence 1 (SEQ ID NO.: 4):
[0098] Ala Thr Thr Ser Thr Gly Asn Ser Ala Arg Phe Val Asn Gln HisLeu
[0099] Cys Gly Ser His Leu Val Glu Ala Leu Tyr Leu Val Cys Gly GluArg
[0100] Gly Phe Phe Tyr Thr Pro Lys Thr Arg Arg Glu Ala Glu Asp LeuGln
[0101] Val Gly Gln Val Glu Leu Gly Gly Gly Pro Gly Ala Gly Ser LeuGln
[0102] Pro Leu Ala Leu Glu Gly Ser Leu Gln Lys Arg Gly Ile Val GluGln
[0103] Cys Cys Thr Ser Ile Cys Ser Leu Tyr Gln Leu Glu Asn Tyr CysAsn
[0104] Proinsulin sequence 1 corresponds to formula II, where
[0105] X is the C-peptide of human insulin (SEQ ID NO.: 3)
[0106] Y is Threonine (B30),
[0107] R 1 Is phenylalanine (B1),
[0108] R 2 Is a peptide with 10 amino acid residues,
[0109] R 3 Is asparagine (A21) and
[0110] A2-A20 is the amino acid seque...
Embodiment 2
[0136] Example 2 (Method of the present invention)
[0137] By fermenting the genetically modified E. coli cells (EP 0489780), a fusion protein having the amino acid sequence shown in Example 1 (proinsulin sequence 1. SEQ ID NO.: 4) was prepared.
[0138] The expressed fusion protein with proinsulin sequence 1 aggregated in E. coli cells to form inclusion bodies. After the fermentation and culture, the cells are separated by centrifugation, and the cells are broken by conventional high-pressure homogenization, and finally the released inclusion bodies of the fusion protein are separated by centrifugation.
[0139] 5 kg of cysteine hydrochloride hydrate was added to the fusion protein aqueous suspension containing 40 kg of the fusion protein (determined by freezing the sample).
[0140] The suspension containing proinsulin sequence 1 (the concentration of the insulin-containing fusion protein is determined to be 50% by HPLC) was dissolved in 550 L of urea solution with a pH of 10...
Embodiment 3
[0145] Example 3 (comparative example, prior art)
[0146] By fermenting the genetically modified E. coli cells (EP 0489780), a fusion protein with the following amino acid sequence was prepared
[0147] Proinsulin sequence 2 (SEQ ID NO.: 5):
[0148] Ala Thr Thr Ser Thr Gly Asn Ser Ala Arg Phe Val Asn Gln HisLeu
[0149] Cys Gly Ser His Leu Val Glu Ala Leu Tyr Leu Val Cys Gly GluArg
[0150] Gly Phe Phe Tyr Thr Pro Lys Thr Arg Arg Glu Ala Glu Asp LeuGln
[0151] Val Gly Gln Val Glu Leu Gly Gly Gly Pro Gly Ala Gly Ser LeuGln
[0152] Pro Leu Ala Leu Glu Gly Ser Leu Gln Lys Arg Gly Ile Val GluGln
[0153] Cys Cys Thr Ser Ile Cys Ser Leu Tyr Gln Leu Glu Asn Tyr CysGly
[0154] Proinsulin sequence 2 corresponds to formula II, where
[0155] X is the C-peptide of human insulin (SEQ ID NO.: 3)
[0156] Y is Threonine (B30),
[0157] R 1 Is phenylalanine (B1),
[0158] R 2 Is a peptide with 10 amino acid residues,
[0159] R 3 Is glycine (A21) and
[0160] A2-A20 is the amino acid seque...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com