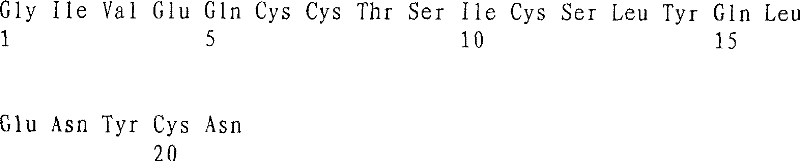Method for obtaining insulin or insulin derivatives with correctly bonded cystine bonds
An insulin derivative, cystine bond technology, applied in microorganism-based methods, biochemical equipment and methods, insulin, etc., can solve problems such as loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1 (comparative example, prior art)
[0096] The genetically modified E. coli cells were fermented (EP 0 489 780) to prepare a fusion protein with the following amino acid sequence.
[0097] Proinsulin sequence 1 (SEQ ID NO.: 4):
[0098] Ala Thr Thr Ser Thr Gly Asn Ser Ala Arg Phe Val Asn Gln HisLeu
[0099] Cys Gly Ser His Leu Val Glu Ala Leu Tyr Leu Val Cys Gly GluArg
[0100] Gly Phe Phe Tyr Thr Pro Lys Thr Arg Arg Glu Ala Glu Asp LeuGln
[0101] Val Gly Gln Val Glu Leu Gly Gly Gly Pro Gly Ala Gly Ser LeuGln
[0102] Pro Leu Ala Leu Glu Gly Ser Leu Gln Lys Arg Gly Ile Val GluGln
[0103] Cys Cys Thr Ser Ile Cys Ser Leu Tyr Gln Leu Glu Asn Tyr CysAsn
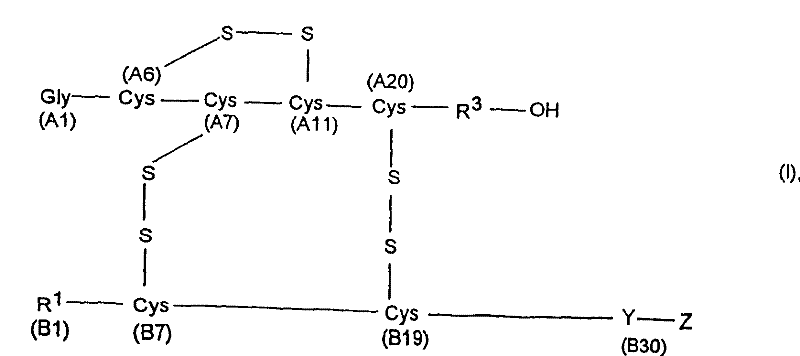
[0104] Proinsulin sequence 1 corresponds to formula II, where
[0105] X is the C-peptide of human insulin (SEQ ID NO.: 3)
[0106] Y is Threonine (B30),
[0107] R 1 Is phenylalanine (B1),
[0108] R 2 Is a peptide with 10 amino acid residues,
[0109] R 3 Is asparagine (A21) and
[0110] A2-A20 is the amino acid sequence of human...
Embodiment 2
[0136] Example 2 (Method of the present invention)
[0137] By fermenting the genetically modified E. coli cells (EP0489780), a fusion protein having the amino acid sequence shown in Example 1 (proinsulin sequence 1. SEQ ID NO.: 4) was prepared.
[0138] The expressed fusion protein with proinsulin sequence 1 aggregated in E. coli cells to form inclusion bodies. After the fermentation and culture, the cells are separated by centrifugation, and the cells are broken by conventional high-pressure homogenization, and finally the released inclusion bodies of the fusion protein are separated by centrifugation.
[0139] 5 kg of cysteine hydrochloride hydrate was added to an aqueous suspension of fusion protein containing 40 kg of fusion protein (determined by freeze-dried samples).
[0140] The suspension containing proinsulin sequence 1 (the concentration of the insulin-containing fusion protein is determined to be 50% by HPLC) was dissolved in 550 L of urea solution with a pH of 10.2 and...
Embodiment 3
[0145] Example 3 (comparative example, prior art)
[0146] By fermenting the genetically modified E. coli cells (EP0489780), a fusion protein with the following amino acid sequence was prepared
[0147] Proinsulin sequence 2 (SEQ ID NO.: 5):
[0148] Ala Thr Thr Ser Thr Gly Asn Ser Ala Arg Phe Val Asn Gln HisLeu
[0149] Cys Gly Ser His Leu Val Glu Ala Leu Tyr Leu Val Cys Gly GluArg
[0150] Gly Phe Phe Tyr Thr Pro Lys Thr Arg Arg Glu Ala Glu Asp LeuGln
[0151] Val Gly Gln Val Glu Leu Gly Gly Gly Pro Gly Ala Gly Ser LeuGln
[0152] Pro Leu Ala Leu Glu Gly Ser Leu Gln Lys Arg Gly Ile Val GluGln
[0153] Cys Cys Thr Ser Ile Cys Ser Leu Tyr Gln Leu Glu Asn Tyr CysGly
[0154] Proinsulin sequence 2 corresponds to formula II, where
[0155] X is the C-peptide of human insulin (SEQ ID NO.: 3)
[0156] Y is Threonine (B30),
[0157] R 1 Is phenylalanine (B1),
[0158] R 2 Is a peptide with 10 amino acid residues,
[0159] R 3 Is glycine (A21) and
[0160] A2-A20 is the amino acid sequence of human insu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com