A method for determining the glycosylation and terminal modification of immunoglobulin charge variants
An immunoglobulin and charge isomerization technology, applied in the field of kits for glycosylation and terminal modification, can solve the problems of complex sample processing, high cost, long time consumption, etc., and achieve the effect of simple sample processing and improved accuracy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Condition Screening of Immunoglobulin Reduction Method
[0050] 1.1 Investigate the amount of reducing agent DTT
[0051] The effects of four different amounts of DTT on the separation of light and heavy chains were investigated. Take 4 parts of 5 μg antibody protein A, add them to 10 μL 6M guanidine hydrochloride solution respectively, then add 2 μL and 5 μL of 0.1 MDTT solution, and 2 μL and 4 μL of 0.5 MDTT solution respectively, and finally add an appropriate amount of 6M guanidine hydrochloride solution to make the final concentration of DTT respectively 10 mM, 25mM, 50mM and 100mM, and react with the IgG1 protein at 65°C for 45min.
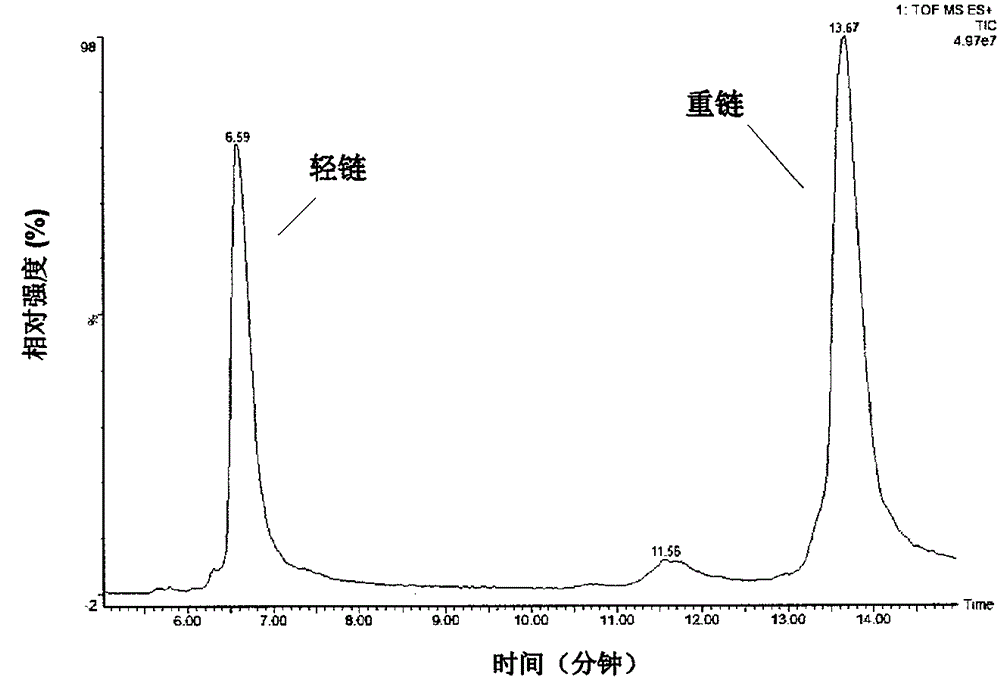
[0052] The light chain and heavy chain obtained in the reaction were separated by C4 reverse ultra-high pressure liquid chromatography, and the liquid phase system used was UPLC (Waters, ACQUITY). Chromatographic column: Waters, ACQUITYUPLCcolumn, BEHC4, 1.7μm (particle size), (Aperture), 2.1×50mm. The chromatographic...
Embodiment 2
[0081] Example 2 Using the UPLC-MS method of the present invention to measure the glycosylation and terminal modification of antibody A and antibody B (IgG1)
[0082] Using optimized reducing conditions (5 μg antibody A was added to 10 μL of 6M guanidine hydrochloride solution, then 2 μL of 0.5 MDTT solution was added, and finally an appropriate amount of 6M guanidine hydrochloride solution was added to make the final concentration of DTT 50 mM, and the reaction was carried out at 65°C for 45 minutes). Example 1.1), ESI-MS detection (consistent with Example 1.1) and normalized data processing (consistent with Example 1.2) to analyze the glycosylation and terminal modification of antibody A and antibody B. The first amino acid at the N-terminal of the light chain and heavy chain of antibody A is glutamine (Gln), which is prone to pyroglutamic acid cyclization; the first amino acid at the N-terminal of the light chain of antibody B is glutamic acid (Glu), which is less likely t...
Embodiment 3
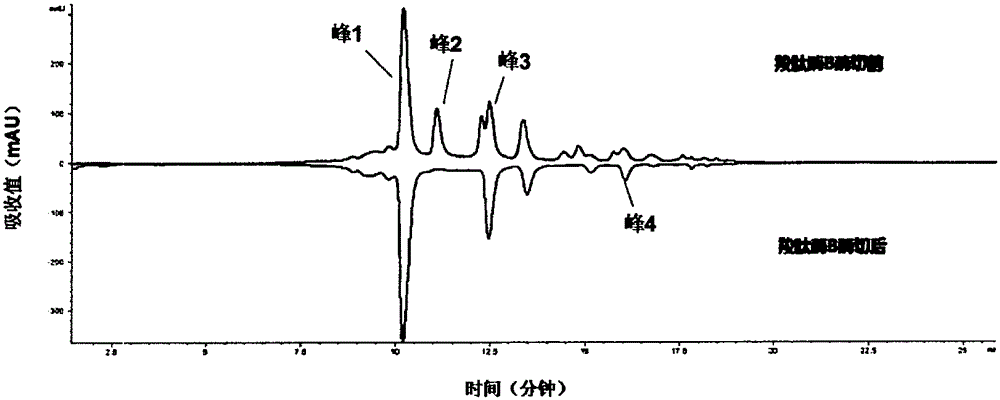
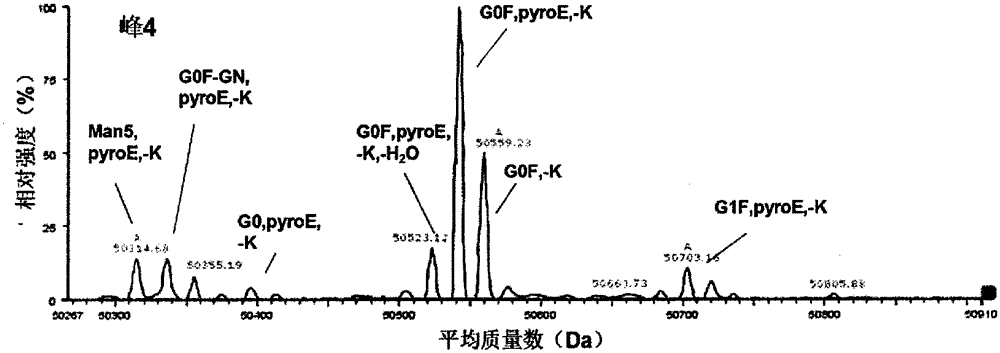
[0085] Example 3 Using the UPLC-MS method of the present invention to determine the glycosylation and terminal modification of the charge variant of antibody A
[0086] Take 200 μg of antibody A, add carboxypeptidase B 1 μg, 4 μg, 10 μg and 20 μg respectively, and react at 37°C for about 3 hours. Then adopt cation exchange chromatography (CEX-HPLC) to analyze; Chromatographic column can adopt DionnexBioLCMAbPacSCX-104 * 250mm, use mobile phase E (20mMMES and 20mMNaCl) and mobile phase F (20mMMES and 200mMNaCl) to carry out gradient elution (0-3min, 10-20%F, 3-25min, 20-50%F, 25-38min, 50-70%F, 38-40min, 70%F, 40-42min, 70-10%F, 42-45min, 10% F). The results show that when the amount of carboxypeptidase B is 1 μg and 4 μg, the enzyme digestion is insufficient, and when the amount is ≥10 μg, the enzyme digestion is sufficient, so 10 μg carboxypeptidase B / 200 μg antibody is preferred. In addition, several parts of 200 μg of antibody A were added to 10 μg of carboxypeptidase B...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com