Preparation method of insulin
A technology for insulin and proinsulin, which is applied in the field of insulin preparation, can solve the problems of uneasy control of the enzymatic digestion process, difficulty in industrial production, inconsistent action sites, etc., and achieve the effect of low cost, simple method and high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
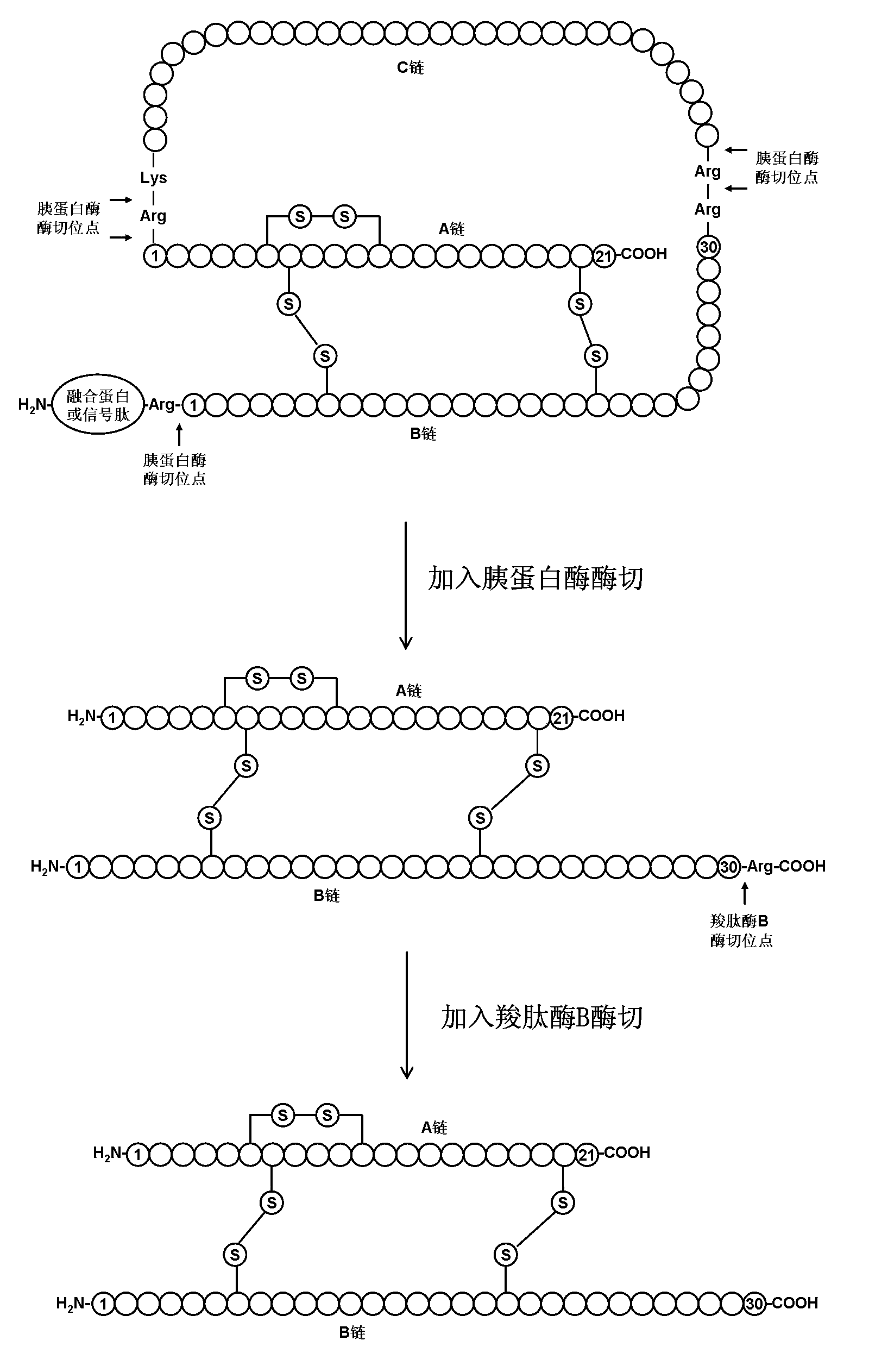
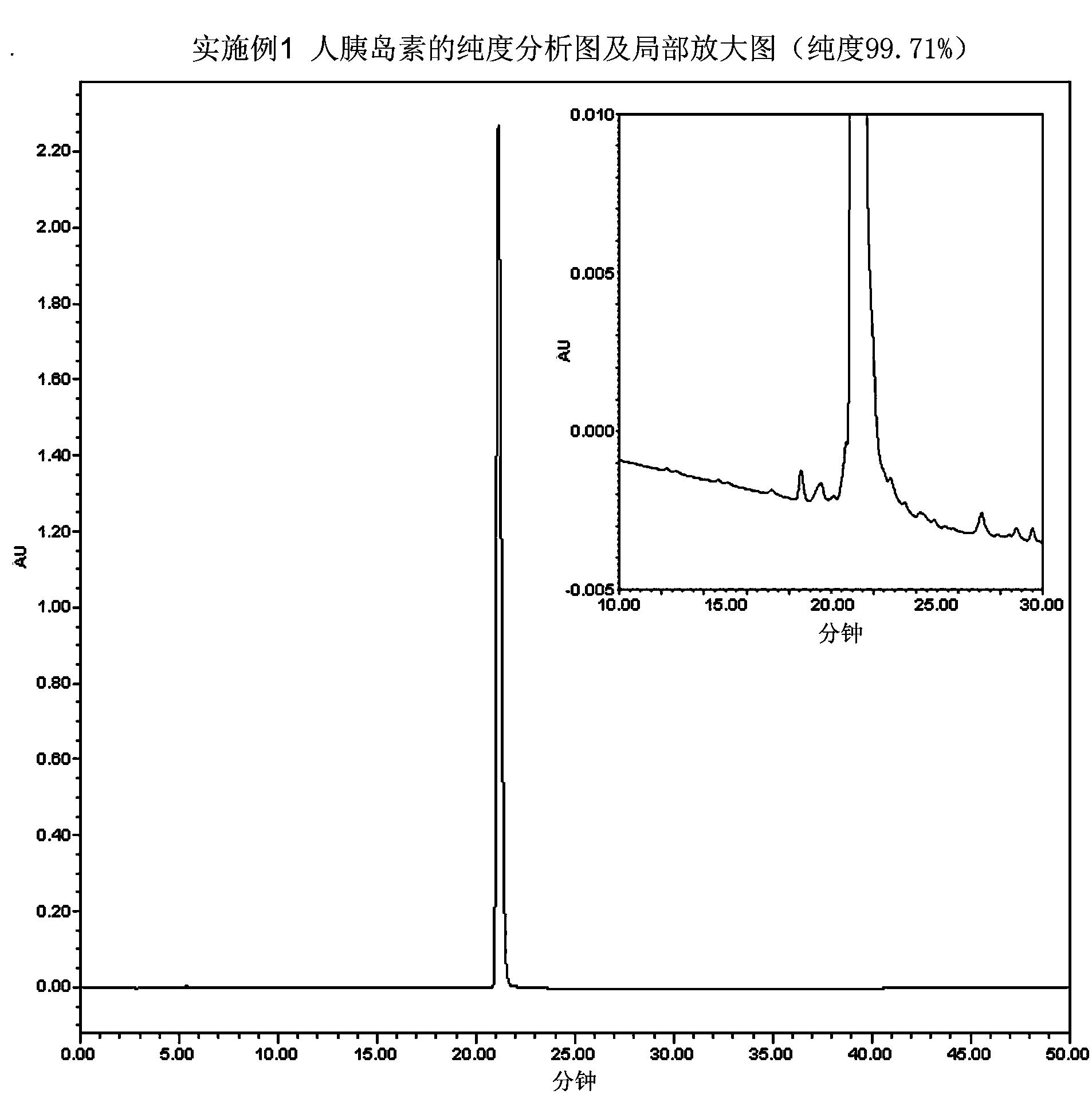
[0040] Embodiment 1 prepares human insulin
[0041] Human proinsulin with the correct conformation was obtained according to steps A1 to C1 in the detailed description of the invention of European patent EP0055945, and prepared into a 1 mg / ml solution. Adjust the pH value of the above solution to 8 with concentrated ammonia water, then add trypsin so that the mass ratio of trypsin to human proinsulin is 1:200, react the mixture at 15°C for 2 hours, and adjust the pH value to 4 with hydrochloric acid To terminate the enzyme cleavage reaction to obtain Arg B31 - human insulin.
[0042] Purify the solution after the above enzyme digestion by hydrophobic chromatography, using phenyl sepharose as the chromatography medium, the eluent is ammonium sulfate solution dissolved in phosphate buffer, gradient 0M-2M, to obtain purified Arg B31 - a human insulin precursor.
[0043] The purified Arg B31 - Human insulin was made into a 1mg / ml solution with 1M Tris buffer at pH 7.5, and t...
Embodiment 2
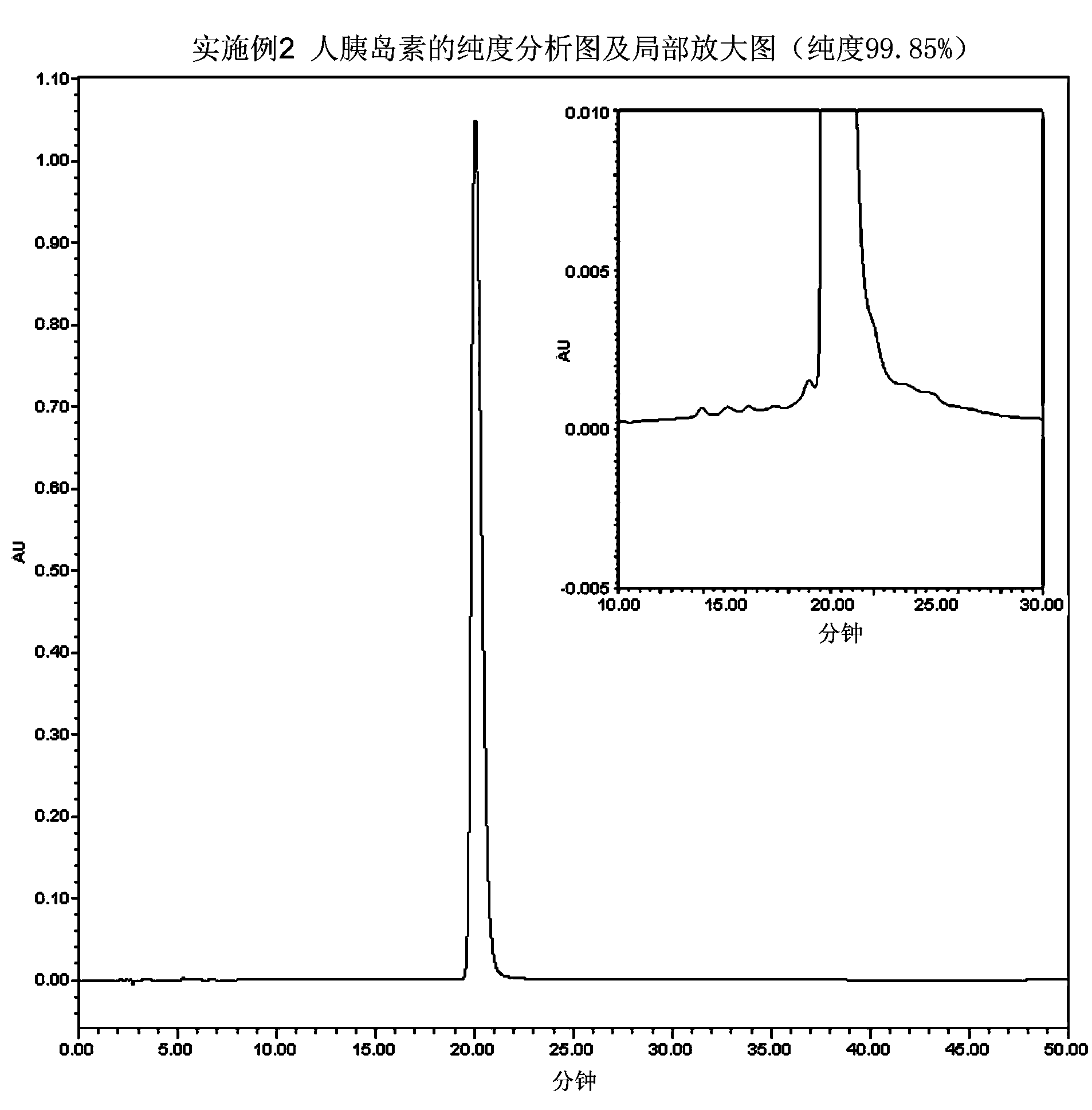
[0046] Embodiment 2 prepares human insulin
[0047] According to Example 5-5.3 of Chinese patent CN98813941.3, the hGH-small proinsulin fusion protein with the correct conformation was obtained and prepared into a 10 mg / ml solution. Adjust the pH value of the above solution to 10.8 with NaOH, then add trypsin, make the mass ratio of trypsin and hGH-small proinsulin fusion protein 1:100, react the mixed solution at 4°C for 5 hours, adjust the pH value with phosphoric acid Adjusted to 3.5 to terminate the enzyme digestion reaction to obtain Arg B31 - human insulin.
[0048] The solution after the above enzyme digestion was subjected to cation exchange chromatography, using fast flow rate CM Sepharose TM As a chromatographic medium, the eluent is NaCl solution dissolved in 10mM citrate buffer, gradient 75mM-225mM, to obtain purified Arg B31 - a human insulin precursor.
[0049] The purified Arg B31 -Human insulin was prepared into a 10mg / ml solution with 50mM Tris-HCl buff...
Embodiment 3
[0052] Embodiment 3 prepares human insulin
[0053] Proinsulin in the correct conformation was obtained according to Example 1 of Chinese Patent CN200610117742.8 (paragraph 30-37 of the description), and prepared into a 15 mg / ml solution. Adjust the pH value of the above solution to 12 with NaOH, then add trypsin so that the mass ratio of trypsin to proinsulin is 1:400, react the mixture at 12°C for 3 hours, adjust the pH value to 4 with phosphoric acid to terminate Enzyme cleavage reaction to get Arg B31 - human insulin.
[0054] The solution after the above digestion was subjected to anion exchange chromatography, using DEAE Sepharose TM As a chromatographic medium, the eluent is NaCl solution dissolved in 0.1M Tris and 50% ethanol solution, gradient 20mM-50mM, to obtain purified Arg B31 - a human insulin precursor.
[0055] The purified Arg B31 -Human insulin is prepared as a 20mg / ml solution, and the pH value is adjusted to 10 with NaOH, and then carboxypeptidase B ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com