Egg-derived bone-strengthening composition
A composition and protein hydrolyzate technology, applied in the direction of drug combination, medical raw materials derived from mammals, animal feed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 (manufacture of egg yolk protein hydrolyzate)
[0046] In 500kg of defatted egg yolk powder obtained by the same method as A, add 2.5 tons of water and Novozymes manufactured by Novozymes Investment Co., Ltd. Protease hydrolysis (trade name, protease derived from bacillus licheniformis) 25 kg, pH 7, 55 ° C for 3 hours to carry out the enzyme reaction, thereafter, at 80 ° C for 15 minutes to inactivate the enzyme, in Centrifuge at a centrifugal force of 3,000g for 20 minutes to remove the precipitate. After filtration, the filtrate is spray-dried to obtain about 140kg of egg yolk protein hydrolyzate.
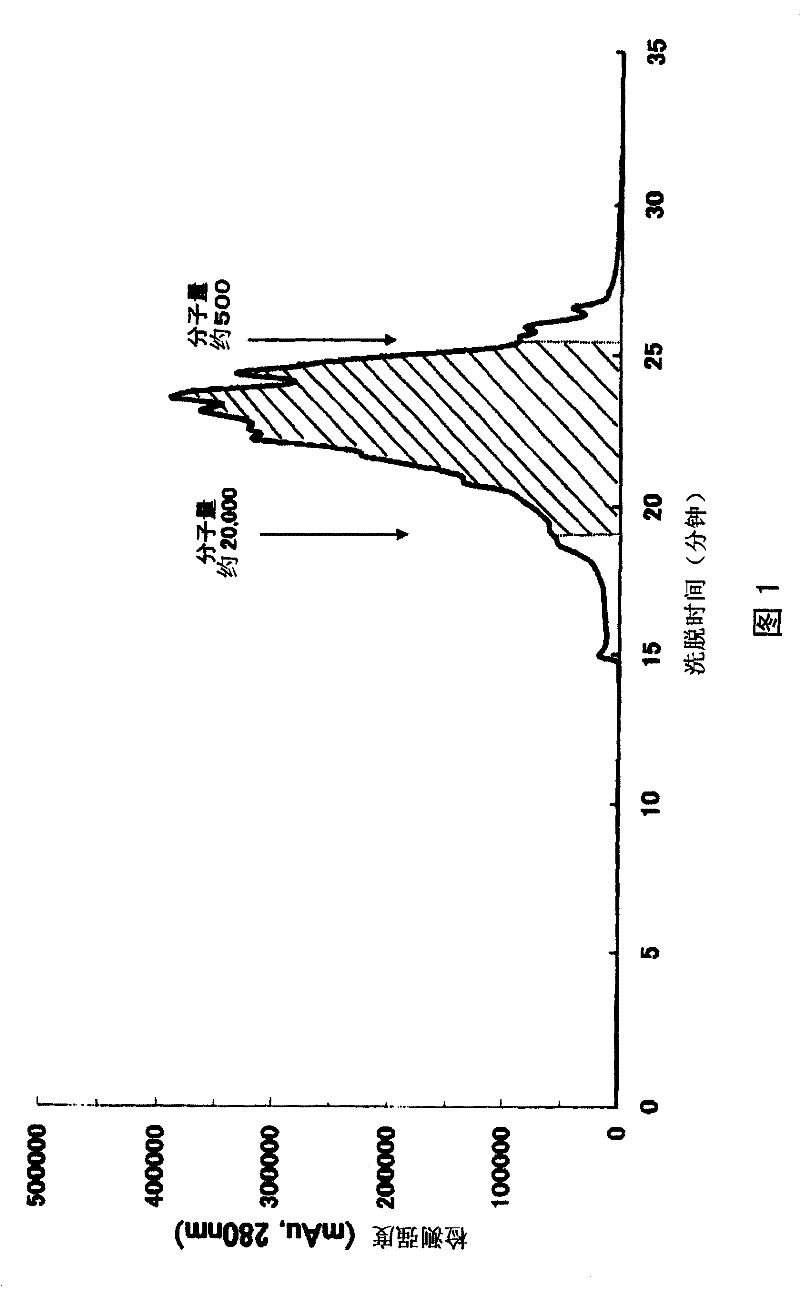
[0047] The egg yolk protein hydrolyzate obtained in Example 1 was subjected to molecular weight analysis by gel permeation chromatography under the following conditions.
[0048] Column: Diol 60 (6.0×300 mm) (trade name, manufactured by YMC Co., Ltd.)
[0049] Eluent: 0.2M potassium phosphate buffer, 0.2M sodium chloride (pH6.9) / acetonitrile (70:30)
[0050] ...
Embodiment 2
[0067] Embodiment 2 (egg yolk protein hydrolyzate)
[0068] Add 3 L of water to 300 g of the defatted egg yolk powder obtained in A, and carry out the enzyme reaction under the enzyme conditions shown in Table 1. After that, after reaching 80°C in a boiling water bath, heat for 10 minutes to inactivate the enzyme, add 30 g and filter auxiliary agent (diatomaceous earth product), filtered with a Buchner funnel, and the obtained filtrate was freeze-dried to obtain 7 kinds of egg yolk protein hydrolyzates.
[0069] [Table 1]
[0070]
[0071] Note: "The area ratio of the specified molecular weight" refers to the area of the portion with a molecular weight of 500 to 20,000 in the total area ratio of protein, peptide, and amino acid when the molecular weight distribution of egg yolk protein hydrolyzate is analyzed by gel permeation chromatography Compare.
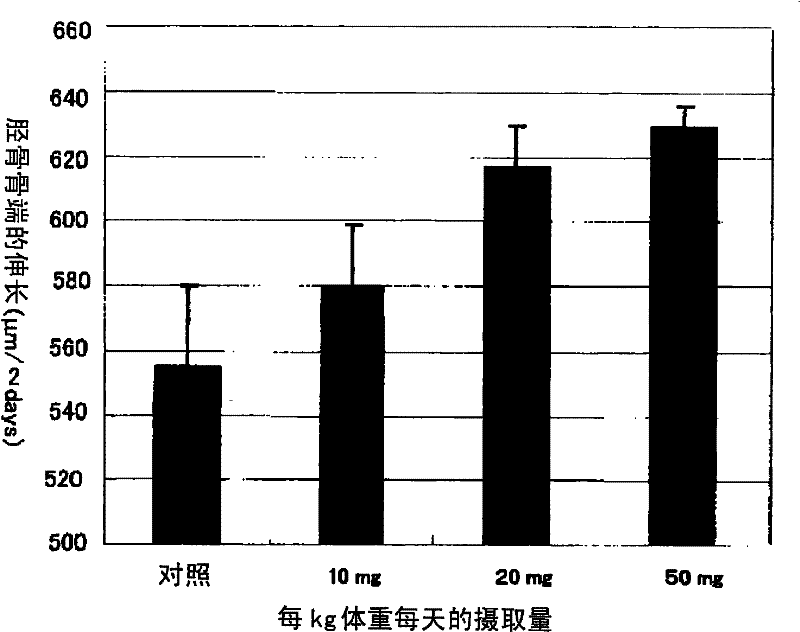
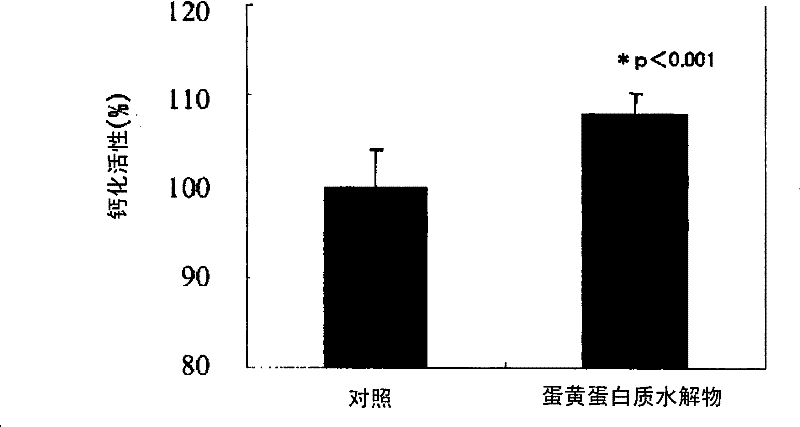
[0072] Test 1 (osteoblast proliferation promoting activity)
[0073] Use α-MEM medium containing 10% FBS at 37°C, 5% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com