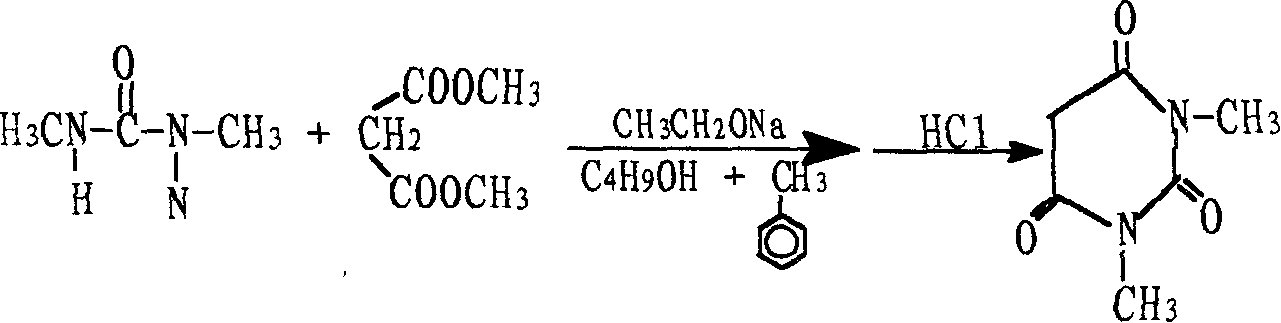Preparation method for 1.3-dimethylbarbituric acid
A technology of dimethyl barbituric acid and dimethyl urea, applied in the field 1, can solve the problems of low yield and high cost of raw materials, and achieve the effects of high yield, low cost of raw materials and excellent product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Add 1.1 mol of dimethyl malonate and 1.0 mol of 1,3-dimethylurea to the reactor, then add 900 grams of solvent n-butanol and toluene and 58 grams of sodium ethoxide as catalyst, and heat to 90-110°C with stirring. The reaction was refluxed for 10 hours. After the reaction, the reactants were cooled to precipitate solids. Dissolve the solids in water, add hydrochloric acid to adjust the pH to 1-2, cool to precipitate crystals, filtered to obtain 132 grams of crude product, recrystallized to obtain 119 grams of pure product, melting point 121.4-123.2°C , The yield is 76%, and the content is 99.7%.
Embodiment 2
[0015] Add 1.2 mol of diethyl malonate and 1.1 mol of 1,3-dimethylurea into the reactor, then add 1150 g of solvent n-butanol and toluene, 72 g of sodium ethoxide as catalyst, and heat to 100-120°C under stirring. The reaction was refluxed for 9 hours. After the reaction, the reactants were cooled to precipitate solids. The solids were collected by filtration. The solids were dissolved in water, adjusted to pH 1-2 with hydrochloric acid, cooled to precipitate crystallization, filtered to obtain 144 grams of crude product, and recrystallized to obtain pure product 130 g, melting point 121.3-123.1°C, yield 75%, content 99.6%.
Embodiment 3
[0017] Add 1.3 mol of dimethyl malonate and 1.2 mol of 1,3-dimethylurea into the reactor, then add 1100 grams of solvent n-butanol and toluene, and 70 grams of sodium ethoxide as catalyst, and heat to 100-120°C under stirring. Reflux the reaction for 10 hours. After the reaction, the reactants were cooled to precipitate solids. The solids were collected by filtration. The solids were dissolved in water, and hydrochloric acid was added to neutralize the pH to 1-2. The crystals precipitated after cooling were filtered to obtain a crude product, 157 grams, and recrystallized 141 grams of pure product was obtained, the yield was 75%, the melting point was 121.3-123.1°C, the yield was 75%, and the content was 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com