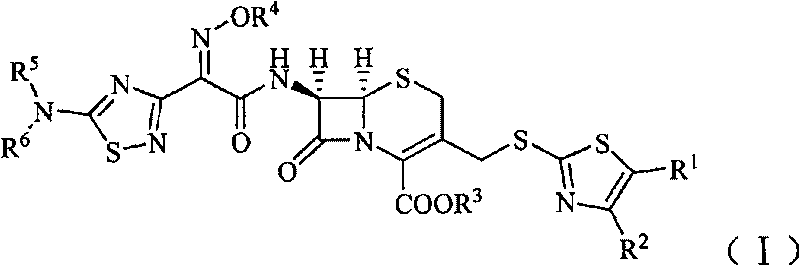
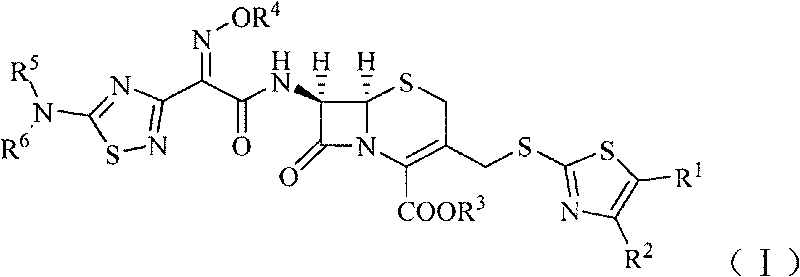
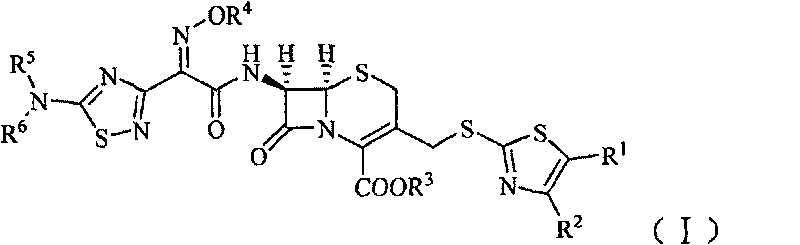
Cephalosporin derivative
A technology of drugs and compounds, applied in the field of medicine, can solve problems such as drug resistance and lack of pharmacological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] Embodiment 1 Preparation method one of compound A and its sodium salt A'
[0138] (1) (6R, 7R)-7-[2-[(5-amino-1,2,4-thiadiazol-3-yl)-2-oximino]acetamido]-3-acetoxymethyl Preparation of yl-5-thia-8-oxo-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
[0139] In the dry reaction bottle, add 27.2g 7-ACA (0.1mol), 40.5g (0.12mol) (Z)-2-(5-amino-1,2,4-thiadiazol-3-yl) - 2-oximinothioacetic acid (S-2-benzothiazole) ester and 500ml of dichloromethane, stir rapidly and control the temperature below 5°C, adjust the pH to 8 with triethylamine, quickly warm up to room temperature, and react 4 Hour. After the reaction was completed, the reaction solution was extracted twice with water (100ml / time), and the pH was adjusted to 2 to 3 with 2mol / L dilute hydrochloric acid after merging the water phases. A white solid was precipitated, filtered, and dried in vacuo to obtain 34.8g of a white solid. The yield was 78.7%.
[0140] (2) (6R, 7R)-7-[2-[(5-amino-1,2,4-thiadiazol 3-yl)2-oxi...
Embodiment 2
[0150] Embodiment 2 Preparation method two of compound A and its sodium salt A'
[0151] (1) (6R, 7R)-7-amino-3-(4-methyl-5-acetoxy-thiazol-2-yl)thiomethyl-8-oxo-5-thia-1-aza Preparation of bicyclo[4.2.0]oct-2-ene-2-carboxylic acid
[0152] Add (6R,7R)-7-amino-3-(acetoxy)methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]octane-2 to the Erlenmeyer flask -Alkene-2-carboxylic acid (ie 7-ACA) 2.8g (0.01mol), water 15ml, shake to make a uniform suspension for use. Add 2.9g (0.015mol) of 2-mercapto-4-methyl-5-thiazoleacetic acid and 40ml of water into a 250ml three-necked flask, add 0.56g (0.014mol) of NaOH solid in batches under stirring, and then use saturated NaHCO 3 Adjust the pH of the solution to 6.4, control the temperature in a water bath at 70°C, then add 7-ACA suspension dropwise and add saturated NaHCO dropwise 3 solution to make the pH of the reaction solution about 6.4, add it in 15 minutes, react for 35 minutes after the addition, then add activated carbon and stir for 10 mi...
Embodiment 3
[0164] Embodiment 3 Compound B and its sodium salt B' preparation method one
[0165] (1) (6R, 7R)-7-[2-[(5-amino-1,2,4-thiadiazol-3-yl)-Z-2-(methoxyimino)]acetamido] - Preparation of 3-acetoxymethyl-5-thia-8-oxo-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
[0166] In a dry reaction flask, sequentially add (6R,7R)-7-amino-3-(acetyloxy)methyl-8-oxo-5-thia-1-azabicyclo[4.2.0] Oct-2-ene-2-carboxylic acid (7-ACA) 27.2g (0.1mol), (Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-Z- 43.9 g (0.12 mol) of 2-(methoxyimino)thioacetic acid (S-2-benzothiazole) ester and 500 ml of dichloromethane, stir rapidly and control the temperature below 5°C, adjust the pH to 8 with triethylamine , rapidly warming up to room temperature, reacted for 4 hours, extracted the reaction solution with water twice (100ml / time) after the completion of the reaction, adjusted the pH to 2-3 with 2mol / L dilute hydrochloric acid after the combined water phase, separated out a white solid, filtered, vacuum After drying...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com