Salt amino acid of ferulic acid
A technology of amino acid salt and ferulic acid, applied in the field of medicine, can solve problems such as poor stability of sodium ferulate, and achieve the effects of easy large-scale industrial production, storage convenience, and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
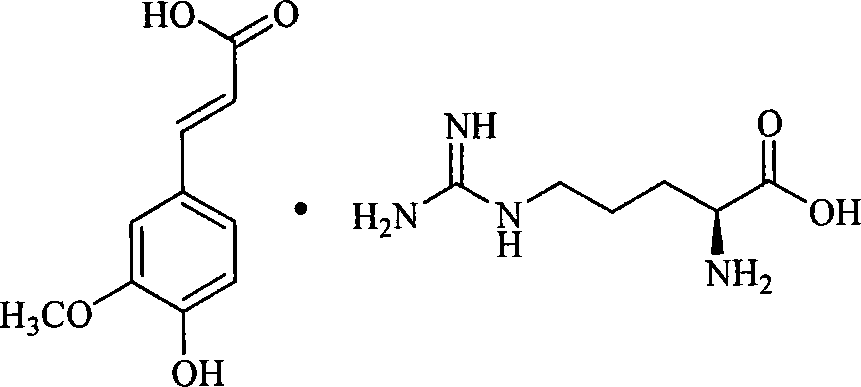
[0078] The preparation of embodiment 1 ferulic acid L-arginine salt
[0079] Put 194g (1mol) of ferulic acid into the reaction flask, add 1000ml of 95% ethanol, 2g of sodium bisulfite, slowly heat up and stir until dissolved, then add 500ml of an aqueous solution containing 174g (1mol) of L-arginine at about 70°C , when the pH of the solution is measured with precision test paper 6.0-7.0, stop adding lye, stir for 10 minutes, add 10 g of activated carbon for decolorization, filter, freeze and crystallize the filtrate for 2 hours, filter, wash the filter cake with absolute ethanol, and dry it in vacuum at 60 ° C to obtain The crude product of ferulic acid L-arginine salt was 348.6g, yield: 94.7%.
[0080] Dissolve the crude product of ferulic acid L-arginine salt in 500ml of deionized water, add 1g of activated carbon, stir and decolorize at 70°C for 15 minutes, filter, freeze the filtrate to 5-10°C, slowly add 1500ml of acetone under stirring, and precipitate A large number...
Embodiment 2
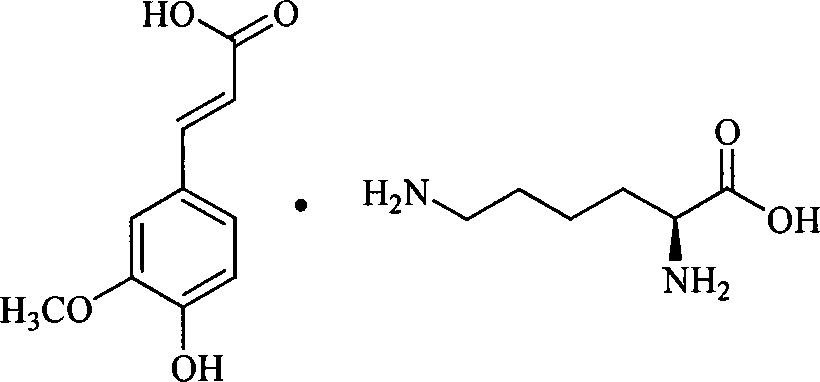
[0084] The preparation of embodiment 2 ferulic acid L-lysine salt
[0085] Refer to Example 1 for specific operation and feeding ratio, replace L-arginine with L-lysine, and the total yield is 78.3%.
[0086] Elemental analysis (molecular formula: C 16 h 24 N 2 o 6 )
[0087] Measured value: C: 54.37%; H: 7.15%; N: 8.19%;
[0088] Theoretical values: C: 54.46%; H: 7.11%; N: 8.23%.
Embodiment 3
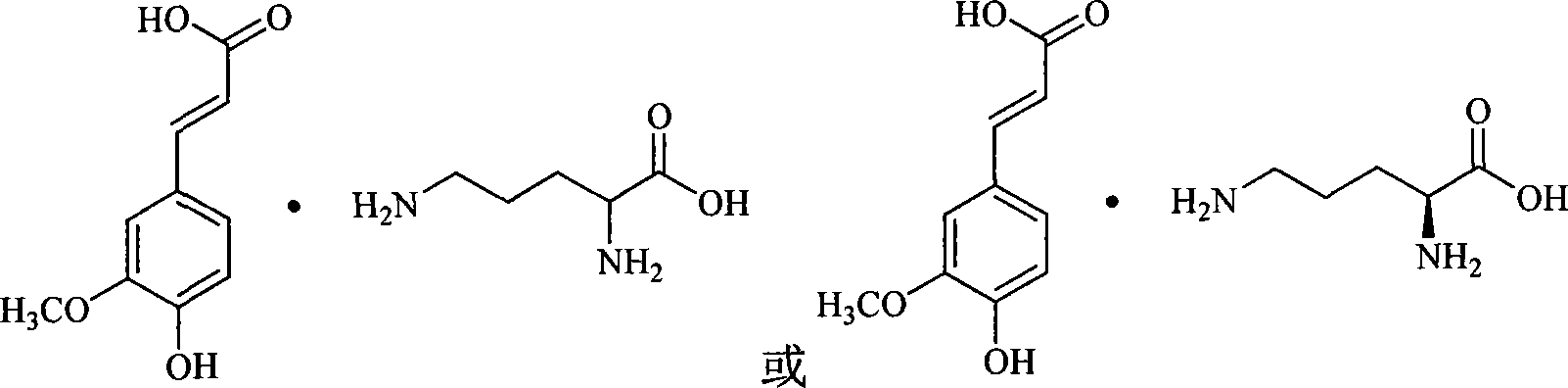
[0089] The preparation of embodiment 3 ferulic acid DL-ornithine salt or ferulic acid L-ornithine salt
[0090] The specific operation and feeding ratio refer to Example 1, and the L-arginine is replaced by DL-ornithine or L-ornithine, and the total yield is 74.2%.
[0091] Elemental analysis (molecular formula: C 15 h 22 N 2 o 6 )
[0092] Measured value: C: 55.13%; H: 6.95%; N: 8.49%;
[0093] Theoretical value: C: 55.21%; H: 6.79%; N: 8.58%;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com