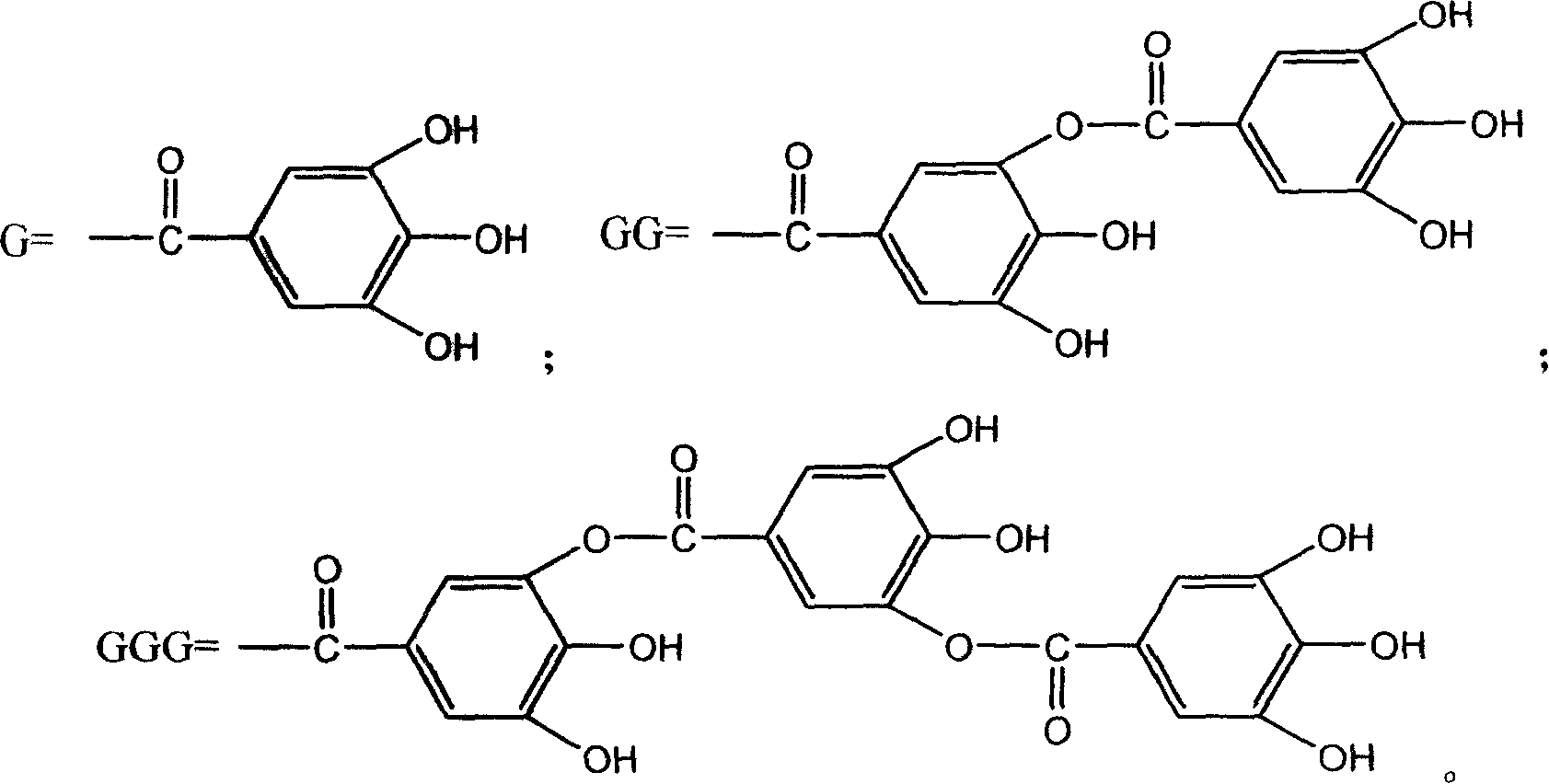
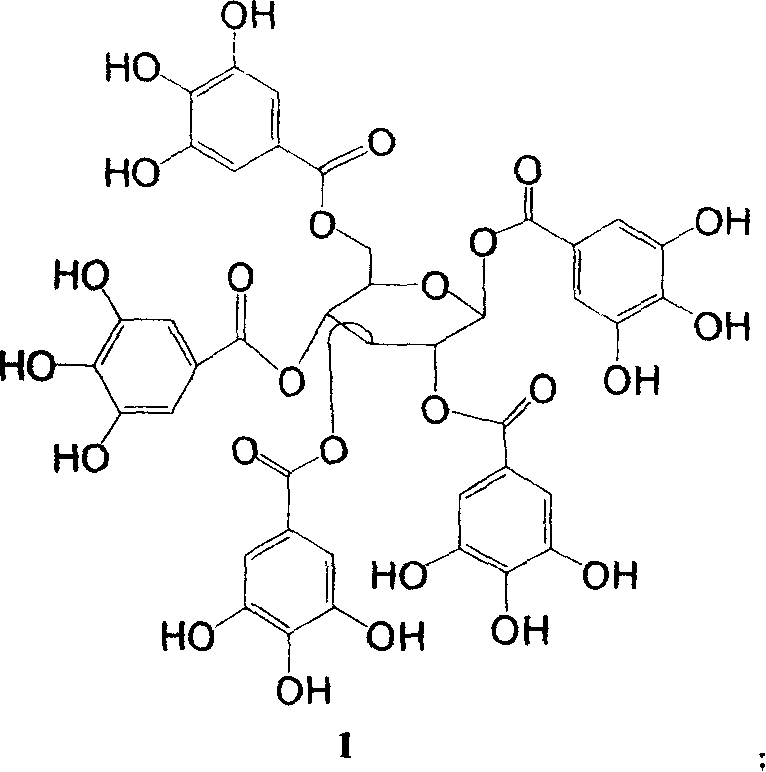
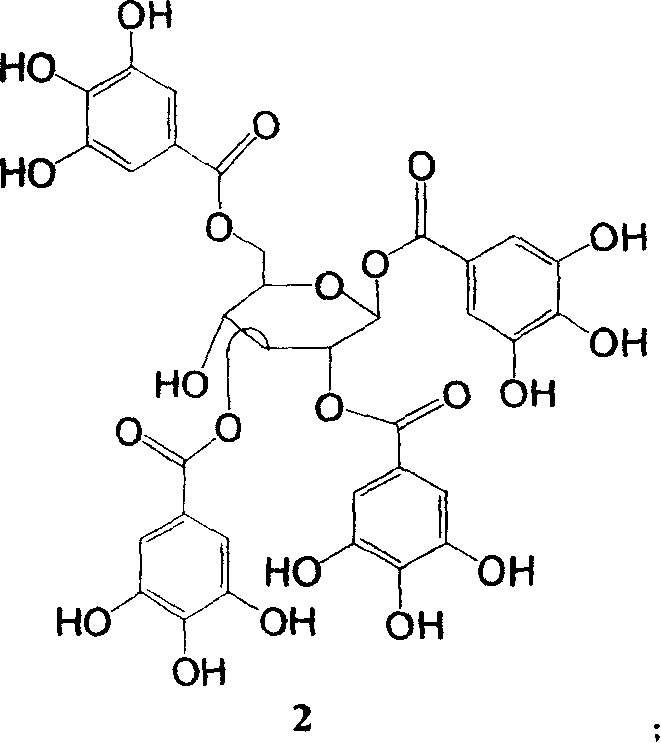
New use of polyhydroxy galloyl-beta-D-glucose derivatives
A technology of glucose derivatives and galloyl group, applied in the field of medicine, can solve problems such as no anti-Helicobacter pylori and the like, and achieve the effects of good anti-Helicobacter pylori activity, low toxic and side effects, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1 prepares compound 2 by geranium
[0064] Take 2000 g of Geranium dahuricum, crush it, add 8 times the amount of 40% ethanol, and reflux three times for 2 hours each time. Filter off the medicinal residues and combine the extracts for 3 times, concentrate under reduced pressure to obtain 280g of extract, add an appropriate amount of water to dissolve, and then use petroleum ether (500ml × 4), ethyl acetate (500ml × 4), n-butanol (500ml × 4 ) for extraction. The ethyl acetate extracts were combined, concentrated to dryness under reduced pressure, applied to a silica gel column in batches (100-200 mesh silica gel G for silica gel), followed by chloroform, chloroform:methanol=10:1, chloroform:methanol=8:1, Chloroform:methanol=5:1, chloroform:methanol=2:1, methanol was eluted and detected by thin layer chromatography. The chloroform:methanol=5:1 eluents were combined and concentrated to dryness. The resultant was repeatedly chromatographed on a gel column (Se...
Embodiment 2
[0065] Embodiment 2 prepares compound 1 and 5 by gallnut
[0066] After crushing 500 g of Galla chinensis, 2000 ml of 50% ethanol-water solution was cold soaked at room temperature to extract three times, soaked for 24 hours each time, and the extracted filtrate was concentrated under reduced pressure to evaporate ethanol to obtain 260 g of crude extract. The crude extract was extracted sequentially with petroleum ether (500ml×4), chloroform (500ml×4), ethyl acetate (500ml×4), and n-butanol (500ml×4), and divided into several parts. The ethyl acetate parts were combined, concentrated and evaporated to dryness, and applied to a gel column (Sephadex LH-20) in batches, followed by water, methanol: water = 2: 8, methanol: water = 4: 6, methanol: water = 6: 4 , methanol: water = 8: 2, methanol, acetone for gradient elution, and detection by thin layer chromatography to obtain 6 components.
[0067] The 6 components were subjected to polyamide column decompression column chromatogr...
Embodiment 3
[0068] Embodiment 3 prepares compound 3 by Rhodiola angustifolia
[0069] 3kg of Rhodiola angustifolia was crushed, soaked and extracted with 10000ml 80% ethanol three times, soaked for one week each time, the crude extract was concentrated under reduced pressure, and after the ethanol was evaporated, 300g of extract was obtained, dissolved in appropriate amount of water, and then washed with petroleum ether (600ml×5 ), chloroform (600ml×5), ethyl acetate (600ml×5), n-butanol (600ml×5) extraction. The organic layer was evaporated to dryness under reduced pressure to obtain 23 g of petroleum ether, 17 g of chloroform, 48 g of ethyl acetate, and 100 g of n-butanol. Ethyl acetate and n-butanol were partly applied to a polyamide column, and the obtained fraction was further purified by means of gel column chromatography to obtain pure compound 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com