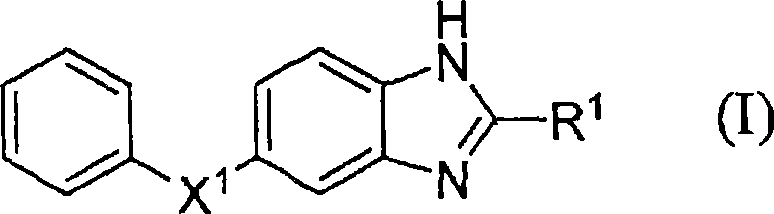
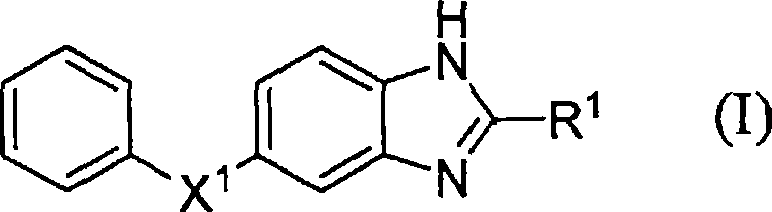
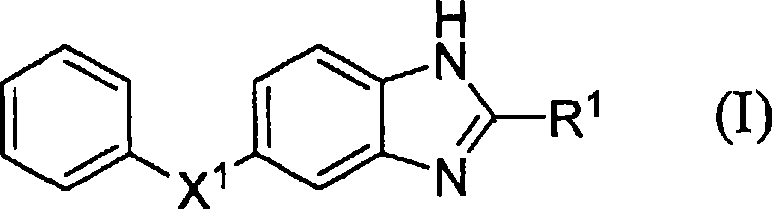
Benzoimidazole compound capable of inhibiting prostaglandin D synthetase
A technology of benzimidazole and compound is applied in the field of medicine in which novel benzimidazole compound or its salt is an active ingredient, and can solve the problems of susceptibility to infection, insufficient drug effect, and no
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0363] 5-Benzoyl-2-(2,4-dimethylfuran-3-yl)-benzimidazole (1)
[0364]
[0365] To a solution of 3,4-diaminobenzophenone (43mg, 0.19mmol) in methanol (3ml) was added 2,4-dimethylaminofuran-3-carboxylic acid (30mg, 0.21mmol) and 4-(4, 6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (65 mg, 0.23 mmol), stirred overnight, and distilled off the solvent under reduced pressure. Chloroform / methanol (7:1) and saturated aqueous sodium carbonate were added to the residue and stirred for 30 minutes, then extracted with chloroform / methanol at the same ratio, and the organic layer was washed with saturated brine and dried over anhydrous magnesium sulfate.
[0366] The residue obtained by distilling off the solvent under reduced pressure was purified by medium-pressure silica gel flash column chromatography (chloroform:methanol=99:1). The resulting condensate was dissolved in acetic acid (4 ml), stirred overnight at 80° C., then left to cool to room temperature, and the solv...
Embodiment 2
[0370] 4-(5-Benzoylbenzimidazol-2-yl)-3,5-dimethylfuran-2-carboxamide (2)
[0371] Example 2(1)
[0372] 3,5-Dimethyl-4-ethoxycarbonylfuran-2-carboxylic acid (raw material for 2)
[0373]
[0374] Dissolve ethyl 2,4-dimethyl-5-formylfuran-3-carboxylate (1.78g, 9.1mmol) known in the literature in a mixture of acetic acid (32ml) and water (8ml), add Sulfamic acid (1.19g, 12.2mmol), the mixture was cooled to 0°C with an ice bath. Sodium chlorite was added and stirred for 2 hours, then water was added to the system, and the precipitated solid was filtered to obtain 3,5-dimethyl-4-ethoxycarbonylfuran-2-carboxylic acid (1.01g, 52%) , as a white solid.
[0375] 1 H-NMR (CDCl 3 ): δ (ppm) 1.38 (t, J = 7.3Hz, 3H), 2.56 (s, 3H), 2.64 (s, 3H), 4.33 (q, J = 7.3Hz, 2H).
[0376] Example 2(2)
[0377] 3,5-Dimethyl-4-ethoxycarbonylfuran-2-carboxamide (raw material for 2)
[0378]
[0379] To the 3,5-dimethyl-4-ethoxycarbonylfuran-2-carboxylic acid (250mg, 1.18mmol) solution obt...
Embodiment 3
[0390] (4-(5-benzoylbenzimidazol-2-yl)-3,5-dimethyl-2-furylcarbonyl)pyrrolidine (3)
[0391] Example 3(1)
[0392] (3,5-Dimethyl-4-ethoxycarbonyl-2-furylcarbonyl)pyrrolidine (raw material for 3)
[0393]
[0394] According to Example 2(2), by using pyrrolidine instead of 28% ammonia water, (3,5-dimethyl-4-ethoxycarbonyl-2-furylcarbonyl)pyrrolidine (89%) was obtained as a white solid .
[0395] 1 H-NMR (CDCl 3 ): δ (ppm) 1.37 (t, J = 7.3Hz, 3H), 1.92 (br, 4H), 2.49 (s, 3H), 2.57 (s, 3H), 3.62-3.73 (m, 4H), 4.31 ( q, J = 7.3 Hz, 2H).
[0396] Example 3(2)
[0397] (4-(5-benzoylbenzimidazol-2-yl)-3,5-dimethyl-2-furylcarbonyl)pyrrolidine (3)
[0398]
[0399] According to Example 2(3), by using (3,5-dimethyl-4-ethoxycarbonyl-2-furylcarbonyl)pyrrolidine instead of 3,5-dimethyl-4-ethoxycarbonylfuran-2 -carboxamide to give (4-(5-benzoylbenzimidazol-2-yl)-3,5-dimethyl-2-furylcarbonyl)pyrrolidine (59%) as a pale yellow solid.
[0400] Melting point: 112-114°C
[0401] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com