Panax notoginseng saponins freeze-dried powder injection and preparing method thereof
A technology for total saponins of Panax notoginseng and freeze-dried powder injection, applied in the field of medicine, can solve the problems of poor appearance and properties, influence product promotion and use, unsatisfactory formability, etc., and achieves improved appearance properties, strong aesthetics, and anti-vibration. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
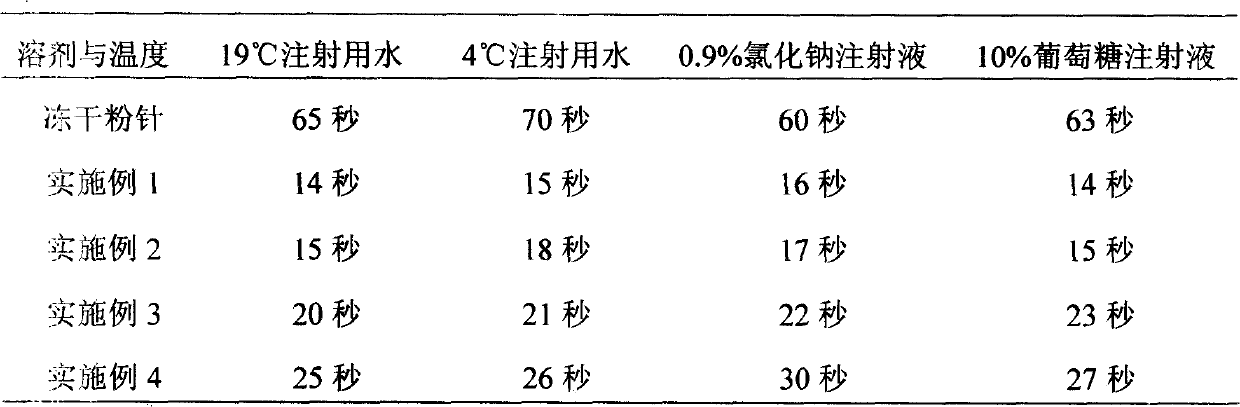
[0051] Raw material formula: (prepared 10,000 sticks, product specifications: each stick contains 0.1g of Panax notoginseng saponins)
[0052] Panax notoginseng saponins 1.0kg
[0053] Mannitol 0.1kg
[0055] Preparation:
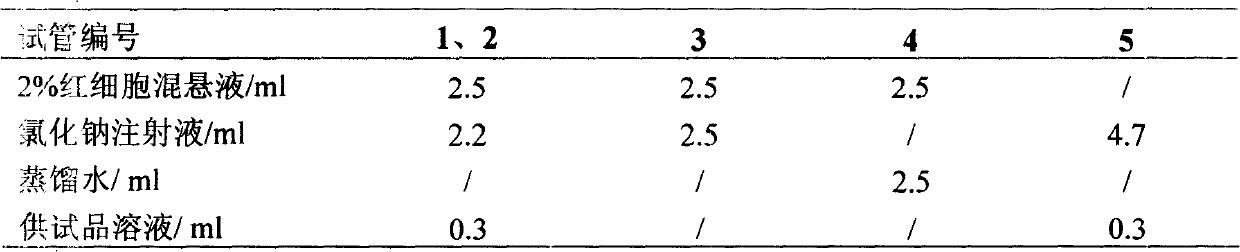
[0056] Under the production conditions of freeze-dried powder injection conforming to the "Good Manufacturing Practice for Drugs" (hereinafter referred to as GMP), take 1.0kg of qualified Panax notoginseng saponins according to the prescription, add 6L of water for injection, stir to dissolve, and then add sodium chloride 0.1kg, after dissolving, add 0.1kg of mannitol, stir to dissolve completely, add water for injection to a total volume of 10L, add activated carbon for needles with a total volume of 1% (W / V) (0.1kg), and absorb for 30 minutes , first use filter paper and sand rod to filter to remove activated carbon, after the filtrate is filtered through a 0.45μm filter membrane, adjust the pH value to 6.5-7.0 with 0.1mo...
Embodiment 2
[0058] Raw material formula: (prepared 10,000 sticks, product specifications: each stick contains 0.1g of Panax notoginseng saponins)
[0059] Panax notoginseng saponins 1.0kg
[0060] Mannitol 0.15kg
[0061] Sodium chloride 0.15kg
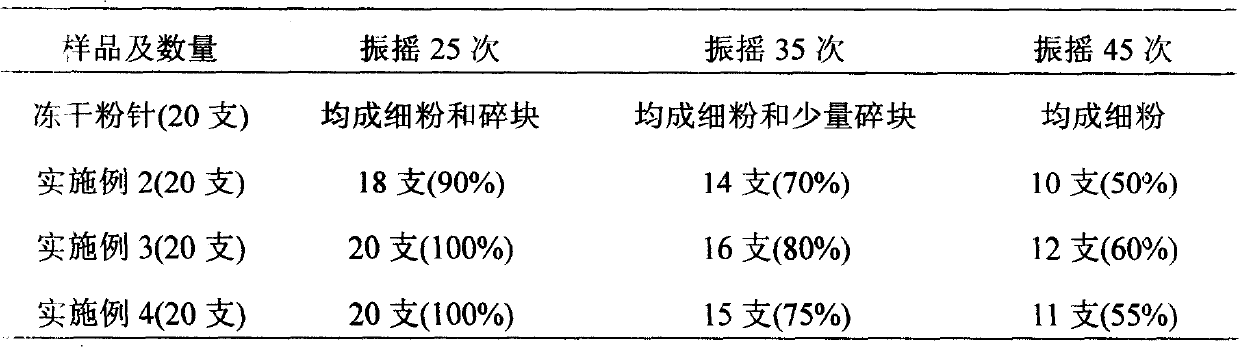
[0062] The technical requirements and operation process of preparation are the same as in Example 1.
Embodiment 3
[0064] Raw material formula: (prepared 10,000 sticks, product specifications: each stick contains 0.1g of Panax notoginseng saponins)
[0065] Panax notoginseng saponins 2.0kg
[0066] Mannitol 0.2kg
[0067] Sodium chloride 0.2kg
[0068] Preparation:
[0069] Under the production conditions of freeze-dried powder injections in compliance with GMP, take 2.0 kg of qualified Panax notoginseng saponins according to the prescription, add 12 L of water for injection, stir to dissolve, then add 0.2 kg of sodium chloride, and add 0.2 kg of mannitol after dissolving, Stir to dissolve completely, add water for injection to a total volume of 20L. Add activated carbon for needles with a total liquid volume of 1% (W / V) (0.2kg), and absorb for 30 minutes. First, filter with filter paper and sand bar to remove activated carbon. / L NaOH to adjust the pH value to 6.5-7.0, and finely filter with a filter membrane below 0.2 μm under the condition of grade 100. The fine filtrate is sent to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com