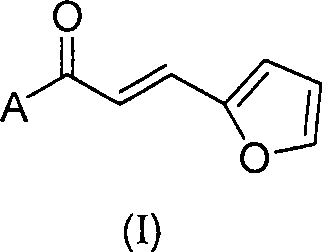
Uses of chalcone synthesis on agricultural chemical
A technology of chalcones and compounds, which is applied in the field of pesticides, and can solve problems such as the application of chalcones in the control of agricultural pests that have not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Compound of Example 1 preparation of
[0021] 4-Fluoroacetophenone (10 mmol, 1.38 g) was dissolved in 10 ml of ethanol, and furfural (10 mmol, 0.96 g) was added. Under the condition of constant stirring, NaOH powder (1 mmol, 0.04 g) was added several times, and stirred evenly. Keep the temperature of the reaction system at 0°C, after the addition is complete, continue the reaction at room temperature for 24 hours, add 100ml of water for dilution, a yellow solid precipitates, filter out the solid, wash it, dry it with infrared, and recrystallize it with absolute ethanol to obtain .
[0022] 1 H-NMR (400MHz, CDCl 3 ): δ6.52-6.53 (1H, m), 6.74 (1H, d, J=3.6), 7.17 (2H, d, J=8.4), 7.43 (1H, d, J=15.2), 7.54 (1H, d, J=1.2), 7.60 (1H, d, J=15.2), 8.07 (2H, d, J=8.0).
Embodiment 2
[0023] Compound of Example 2 preparation of
[0024] 4-Methoxyacetophenone (10 mmol, 1.50 g) was dissolved in 10 ml of ethanol, and furfural (10 mmol, 0.96 g) was added. Under the condition of constant stirring, NaOH powder (1 mmol, 0.04 g) was added several times, and stirred evenly. Keep the temperature of the reaction system at 0°C, after the addition is complete, continue the reaction at room temperature for 24 hours, add 100ml of water for dilution, a yellow solid precipitates, filter out the solid, wash it, dry it with infrared, and recrystallize it with absolute ethanol to obtain .
[0025] 1 H-NMR (400MHz, CDCl 3 ): 3.89 (3H, s) δ6.51-6.52 (1H, m), 6.70 (1H, d, J = 3.2), 6.97 (2H, d, J = 8.8), 7.47 (1H, d, J = 15.2 ), 7.52 (1H, d, J=1.6), 7.34 (1H, d, J=15.2), 8.04 (2H, d, J=8.8).
Embodiment 3
[0026] Compound of Example 3 preparation of
[0027] 2,4-Dichloroacetophenone (10 mmol, 1.88 g) was dissolved in 10 ml of ethanol, and furfural (10 mmol, 0.96 g) was added. Under the condition of constant stirring, NaOH powder (1 mmol, 0.04 g) was added several times, and stirred evenly. Keep the temperature of the reaction system at 0°C, after the addition is complete, continue the reaction at room temperature for 24 hours, add 100ml of water for dilution, a yellow solid precipitates, filter out the solid, wash it, dry it with infrared, and recrystallize it with absolute ethanol to obtain .
[0028] 1 H-NMR (400MHz, CDCl 3 ): 6.51-6.53 (1H, m), 6.72 (1H, d, J=3.6), 7.01 (1H, d, J=15.6), 7.26 (1H, d, J=15.6), 7.34 (1H, dd, J=2.0, J=8.0), 7.43 (1H, d, J=8.0), 7.47 (1H, d, J=2.0), 7.54 (1H, d, J=1.2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com