Method and device for synthesizing six-membered cyclic compound by continuous method
A cyclic compound and continuous method technology, applied in chemical instruments and methods, organic cyclization, organic chemistry, etc., can solve the problems of low reaction conversion rate, low safety performance, high energy consumption, etc., to improve product quality and yield , reaction conversion rate and safety performance improvement, energy consumption reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
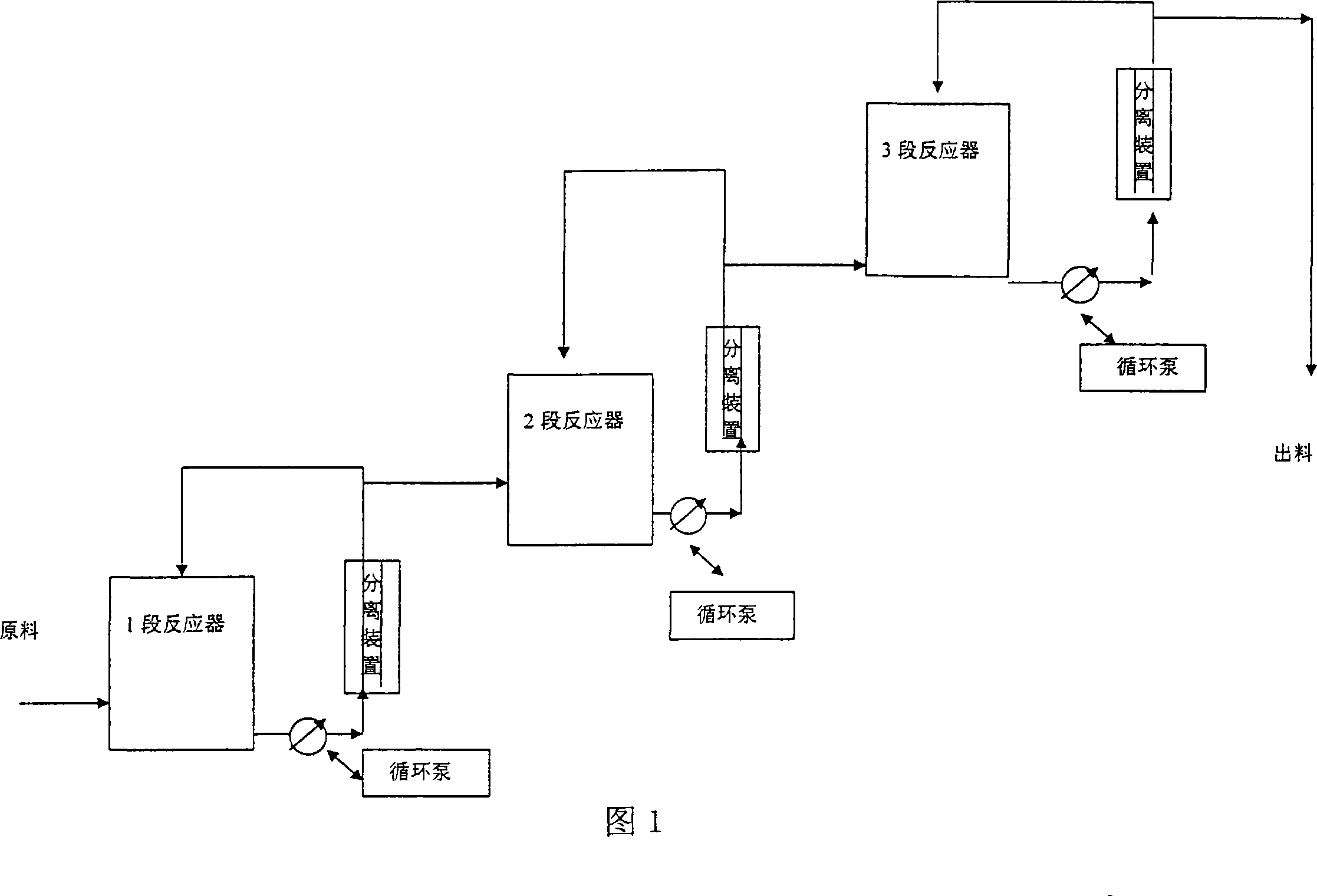
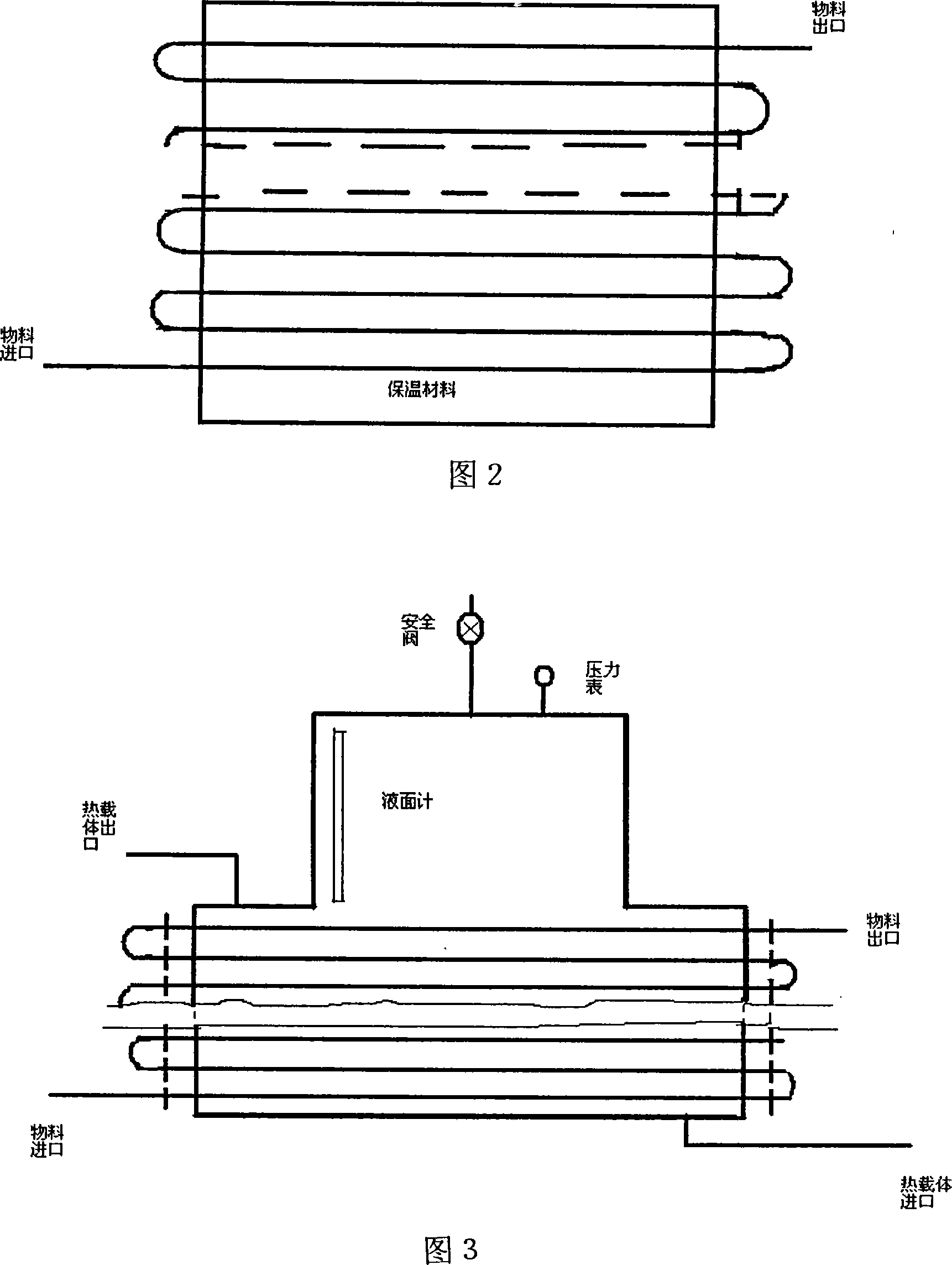
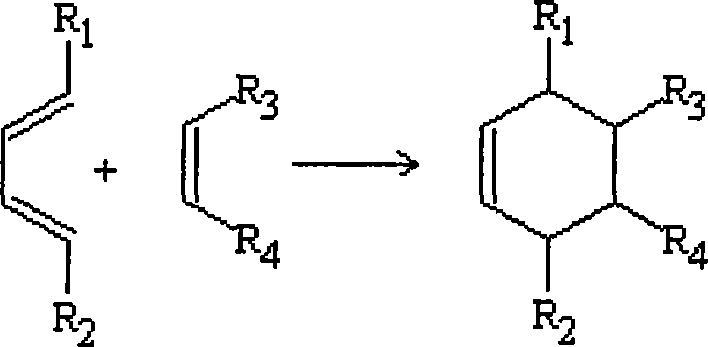
[0029] Acrolein and vinyl methyl ether are mixed according to the molar ratio of 1:1.1, and then pumped into the mixing tank by a high-pressure metering pump at a pressure of 4 MPa, and then enter the first-stage tubular reactor. The temperature is controlled at 130°C, and the circulation ratio is circulation: output =10:1, the discharge part enters the 2-stage reactor, the temperature is controlled at 160°C, the circulation ratio is controlled at 5:1, the discharge part is controlled to enter the 3-stage reactor, and the temperature is controlled at 180°C. The material at the outlet of the third-stage reactor is separated from the raw materials acrolein and butadiene to obtain 2-methoxy-3,4-2H-dihydropyran with a content of 95%, a one-way conversion rate of 90%, and a yield of 92%.
Embodiment 2
[0031] Acrolein and vinyl ethyl ether are mixed according to the molar ratio of 1:1.1, then pumped into the mixing tank by a high-pressure metering pump at a pressure of 5 MPa, and then enter the first-stage tubular reactor. The temperature is controlled at 140° C., and the circulation ratio is circulation: discharge = 15:1, the discharge part enters the second-stage reactor, the temperature is controlled at 160°C, the circulation ratio is controlled at 5:1, the discharge part is controlled to enter the third-stage reactor, the temperature is controlled at 200°C, and the material at the outlet of the third-stage reactor passes through The raw materials of acrolein and butadiene are separated to obtain 2-ethoxy-3,4-2H-dihydropyran with a content of 92%, a single-pass conversion rate of 88%, and a yield of 91%.
Embodiment 3
[0033] Acrolein and butadiene are mixed according to the molar ratio of 1:1.1, then pumped into the mixing tank by a high-pressure metering pump at a pressure of 3 MPa, and then enter the first-stage tubular reactor. The temperature is controlled at 80-100°C, and the circulation ratio is: circulation: out Material=50:1, the discharge part enters the 2nd section reactor, the temperature is controlled at 100-200 °C, the circulation ratio is controlled at 20:1, the discharge part enters the 3rd section reactor, the temperature is controlled at 200 °C, and the circulation ratio is controlled as 10:1, the material at the outlet of the third-stage reactor is separated from the raw materials acrolein and butadiene to obtain 1,2,5,6-tetrahydrobenzaldehyde with a content of 93%, a single-pass conversion rate of 91%, and a yield of 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com