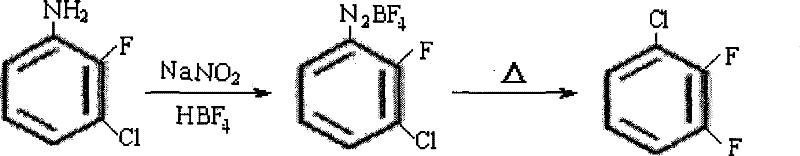
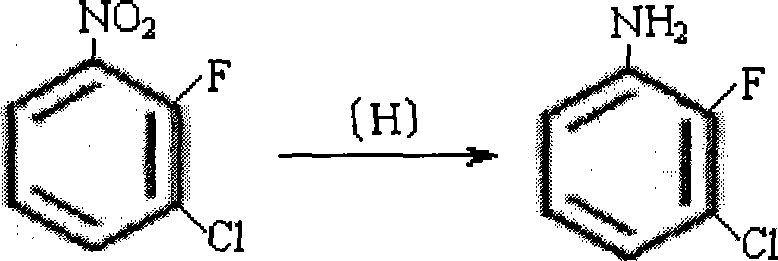Process for synthesizing 2,3-difluoroaniline
A kind of technology of difluoroaniline and synthesis method, applied in 2 fields, can solve problems such as difficult source of raw materials, high price, unsuitable for industrialized production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
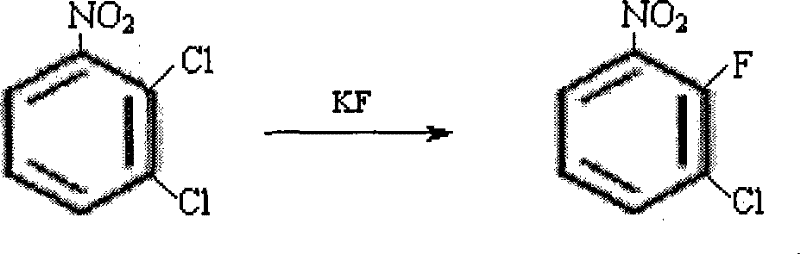
[0005] 1), the preparation of 3-chloro-2-fluoronitrobenzene
[0006] Add 192g (1.0mol) of 2,3-dichloronitrobenzene, 69.6g (1.2mol) of anhydrous KF and 200g of dimethyl sulfoxide (DMSO) into a 500ml four-necked reaction flask for fluorination reaction. Reaction at 170-175°C for 8 hours, cooling to 50°C, filtering, washing the filter cake with 150ml of toluene, combining the filtrates, recovering the solvent by distillation, and then rectifying under reduced pressure to obtain 142.2g of 3-chloro-2-fluoronitrobenzene , yield 81%, content > 99.5%.
[0007] Reaction equation:
[0008]
[0009] 2), the preparation of 3-chloro-2-fluoroaniline
[0010] In a 1000ml pressure-resistant reaction kettle, add 230g (1.31mol) of 3-chloro-2-fluoronitrobenzene, 460ml of methanol, and 7g of Raney nickel catalyst, replace the air in the kettle with nitrogen and replace it with hydrogen for 3 times, and then fill H2 to 1.5MPa, carry out reduction reaction at 35-45°C until no hydrogen is abso...
Embodiment 2
[0024] This embodiment provides a group of embodiments of the fluorination reaction of the present invention. In this embodiment, except for the reaction temperature, other conditions are the same as step "1)" in Example 1. The following table is a statistical table of experimental data:
[0025] DMSO (g)
[0026] 200
[0027] 200
[0028] Conclusion: In the selection of the reaction temperature of the fluorination reaction of the present invention, 170-175° C. has the best effect.
Embodiment 3
[0029] Example 3, the selection of strong polar aprotic solvent in the fluorination reaction of the present invention.
[0030] The fluorination reaction of the present invention is preferably carried out in a strong polar aprotic solvent, and the described strong polar aprotic solvent is preferably with the following substances: N, N-dimethylformamide (DMF), dimethyl Sulfoxide (DMSO), sulfolane or 1,3-dimethyl-2-imidazolidinone (DMI).
[0031] In this example, N, N-dimethylformamide (DMF), sulfolane or 1,3-dimethyl-2-imidazolidinone (DMI) are used to replace the Dimethyl sulfoxide (DMSO), carry out fluorination reaction at 170~175 DEG C respectively, other reaction conditions are identical with the step "1)" among the embodiment 1,
[0032] The following table is the statistical table of the experimental data:
[0033] solvent
[0034] Conclusion: In the synthetic method of the present invention, the effect of choosing dimethyl sulfoxide (DMSO) as the solvent for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com