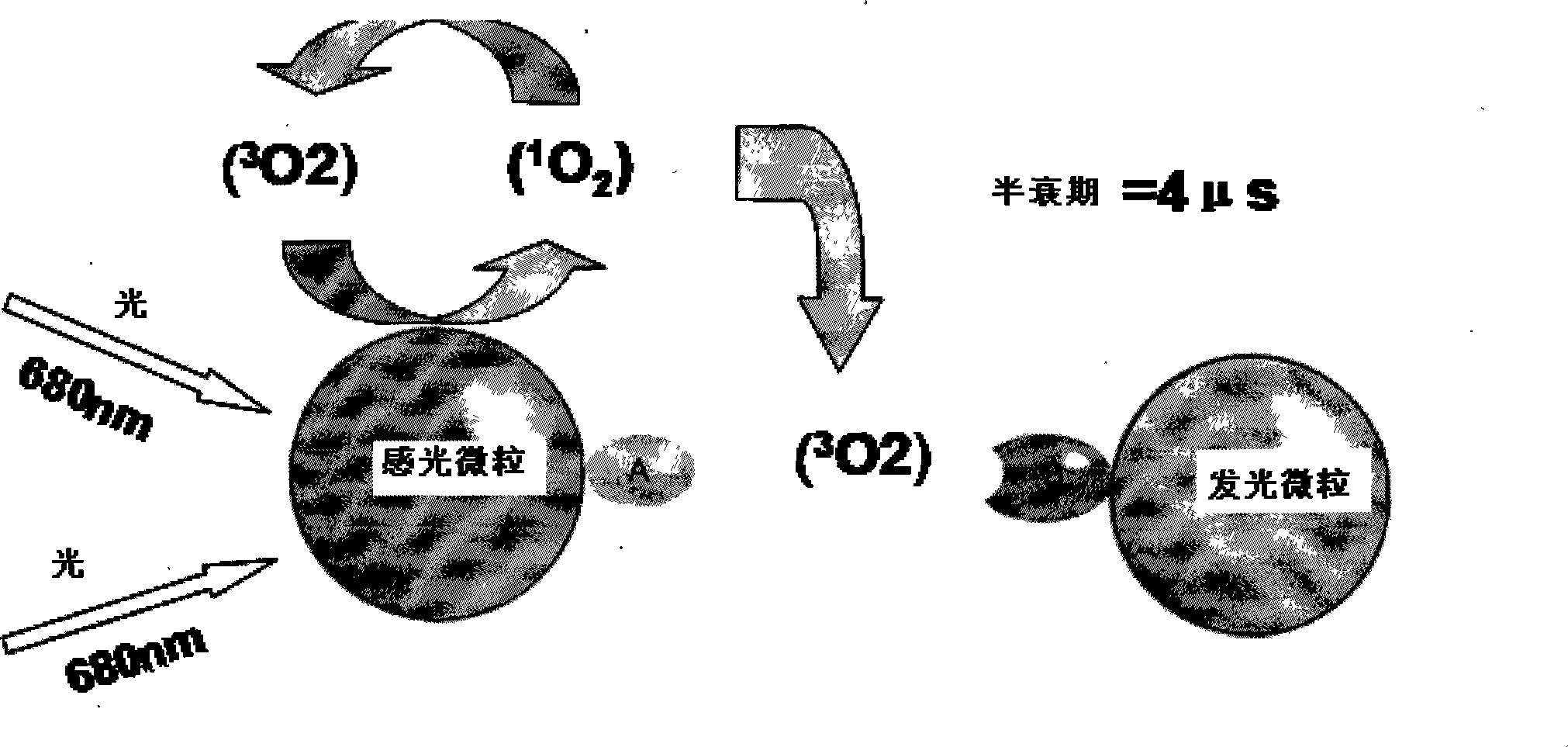
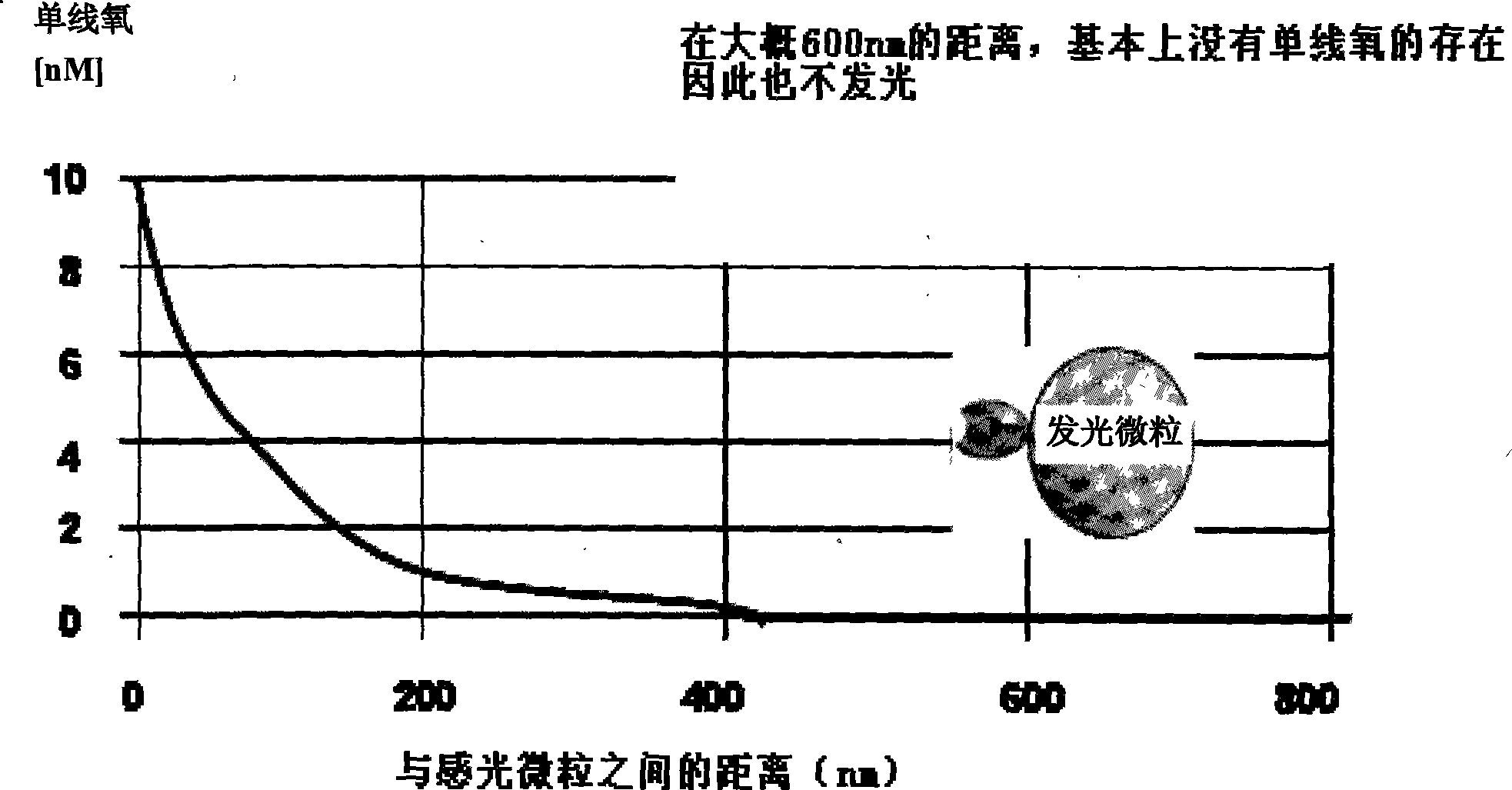
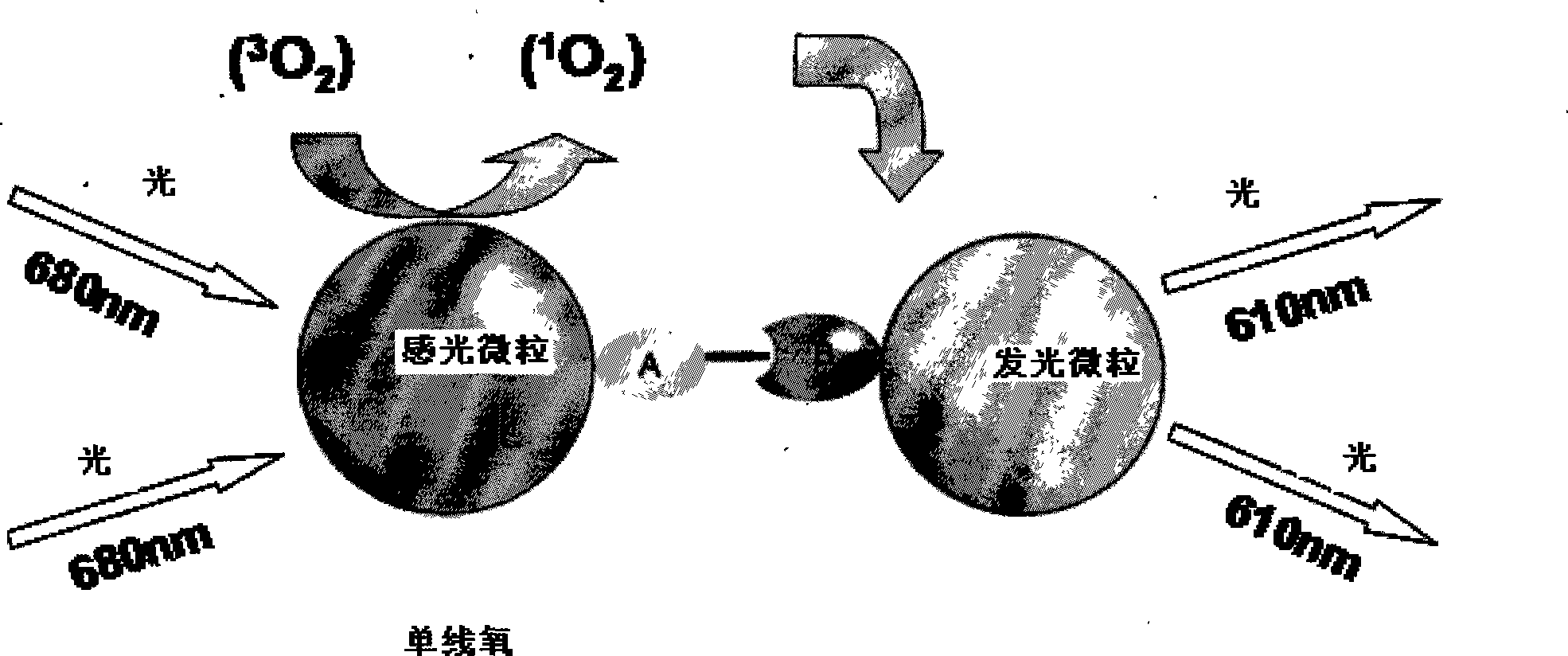Hepatitis B virus e antigen testing corpuscle, preparation and application thereof
A hepatitis B and antigen technology, applied in the field of hepatitis B virus e antigen detection particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1 Preparation of anti-HBe antibody-coated luminescent particles
[0088] Preparation:
[0089] 1) Treatment of luminescent particle suspension: a certain amount of luminescent particles is centrifuged in a high-speed refrigerated centrifuge, the supernatant is discarded, a certain amount of MES buffer is added, and the particles are sonicated until the particles are resuspended, and MES buffer is added to adjust the concentration of luminescent particles To 100mg / ml.
[0090] 2) Antibody treatment: Anti-HBe is dialyzed against 0.05M pH 6.0 MES buffer (hereinafter referred to as MES buffer), and the concentration is measured after the dialysis is completed, and the concentration is adjusted to 8 mg / ml.
[0091] 3) MES buffer, 100mg / ml luminescent particle suspension (MES buffer) and 8mg / ml anti-HBe (MES buffer) and mix at a volume ratio of 1:2:5 and mix quickly , Get the reaction solution.
[0092] 4) Prepare 25mg / ml NaBH with MES buffer 3 The CN solution was added in a ...
Embodiment 2
[0131] Example 2 Preparation of biotin-labeled antibody
[0132] Preparation:
[0133] 1) Antibody treatment: Dialysis anti-HBe to 0.1M NaHCO 3 Solution, measure the antibody concentration and adjust to 1mg / ml.
[0134] 2) Prepare 16.17mg / ml Biotin solution with DMSO.
[0135] 3) Labeling: Take the processed 1mg / ml anti-HBe labeling antibody and the prepared Biotin solution, mix them according to the volume ratio of 10000:54, and mix them quickly. Let stand at 2-8°C for 12-16 hours.
[0136] 4) Dialysis: Dialysis the reacted biotin-labeled antibody against biotin-labeled dialysis buffer (pH 8.00).
[0137] 5) Aspirate and transfer the dialyzed biotinylated antibody to a clean centrifuge tube, and take a sample to determine the antibody concentration. Adjust the concentration of the biotin-labeled antibody that has passed the quality inspection to 0.5 mg / ml.
[0138] The antibody and Biotin are reacted in different ratios and tested:
[0139] Optical signal detection method:
[0140] Add 2...
Embodiment 3
[0143] Example 3 Preparation of avidin-coated photosensitive particles
[0144] Photosensitive particles: use photosensitive particles with a particle size of 220±40nm (PentaTek, USA)
[0145] Preparation:
[0146] a. Processing of photosensitive particle suspension: absorb a certain amount of photosensitive particles and centrifuge in a high-speed refrigerated centrifuge, discard the supernatant, add a certain amount of MES buffer, sonicate on the ultrasonic cell disruptor until the particles are resuspended, and add MES buffer Adjust the concentration of photosensitive particles to 100mg / ml.
[0147] b. Preparation of avidin solution: weigh a certain amount of Avidin, add MES buffer to dissolve it to 8mg / ml.
[0148] c. Mixing: Mix the processed photosensitive particle suspension, 8mg / ml Avidin and MES buffer at a volume ratio of 2:5:1, and mix quickly to obtain a reaction solution.
[0149] d. Reaction: 25mg / ml NaBH prepared with MES buffer 3 The CN solution was added according to th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com