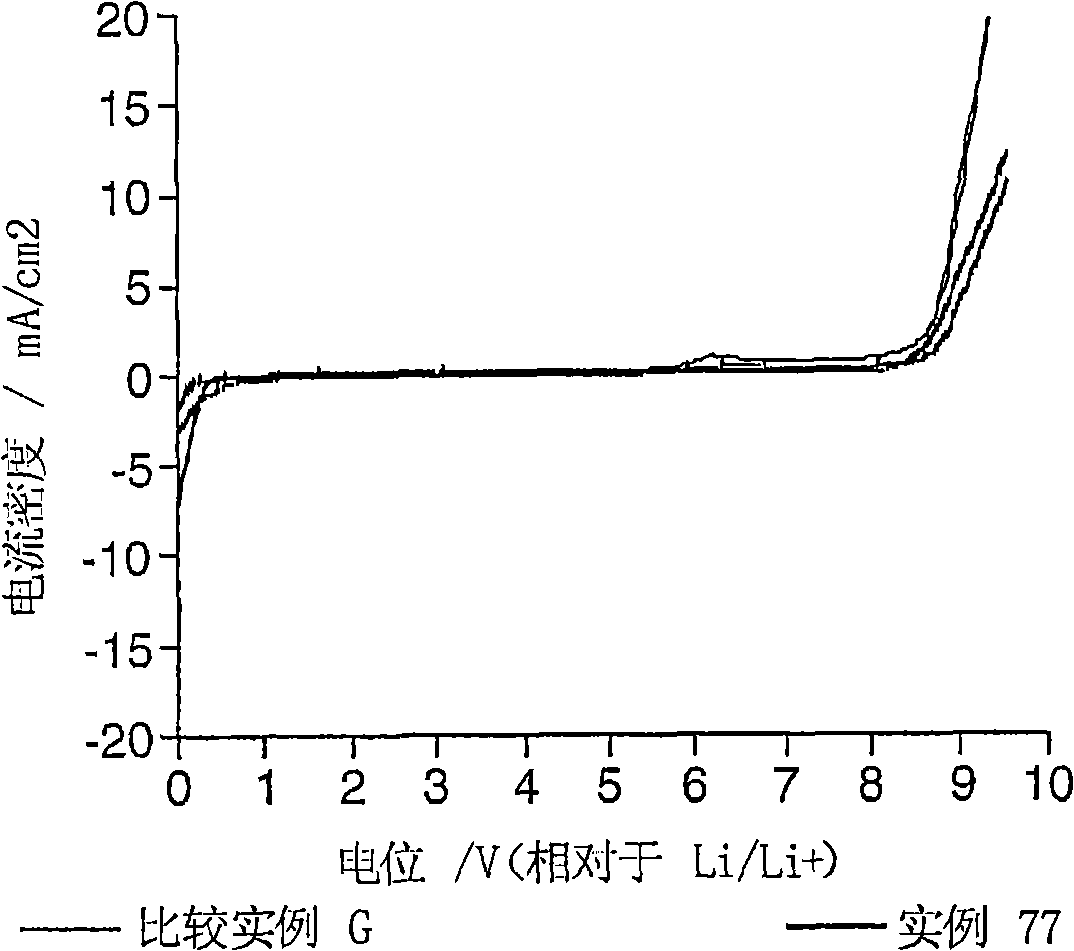
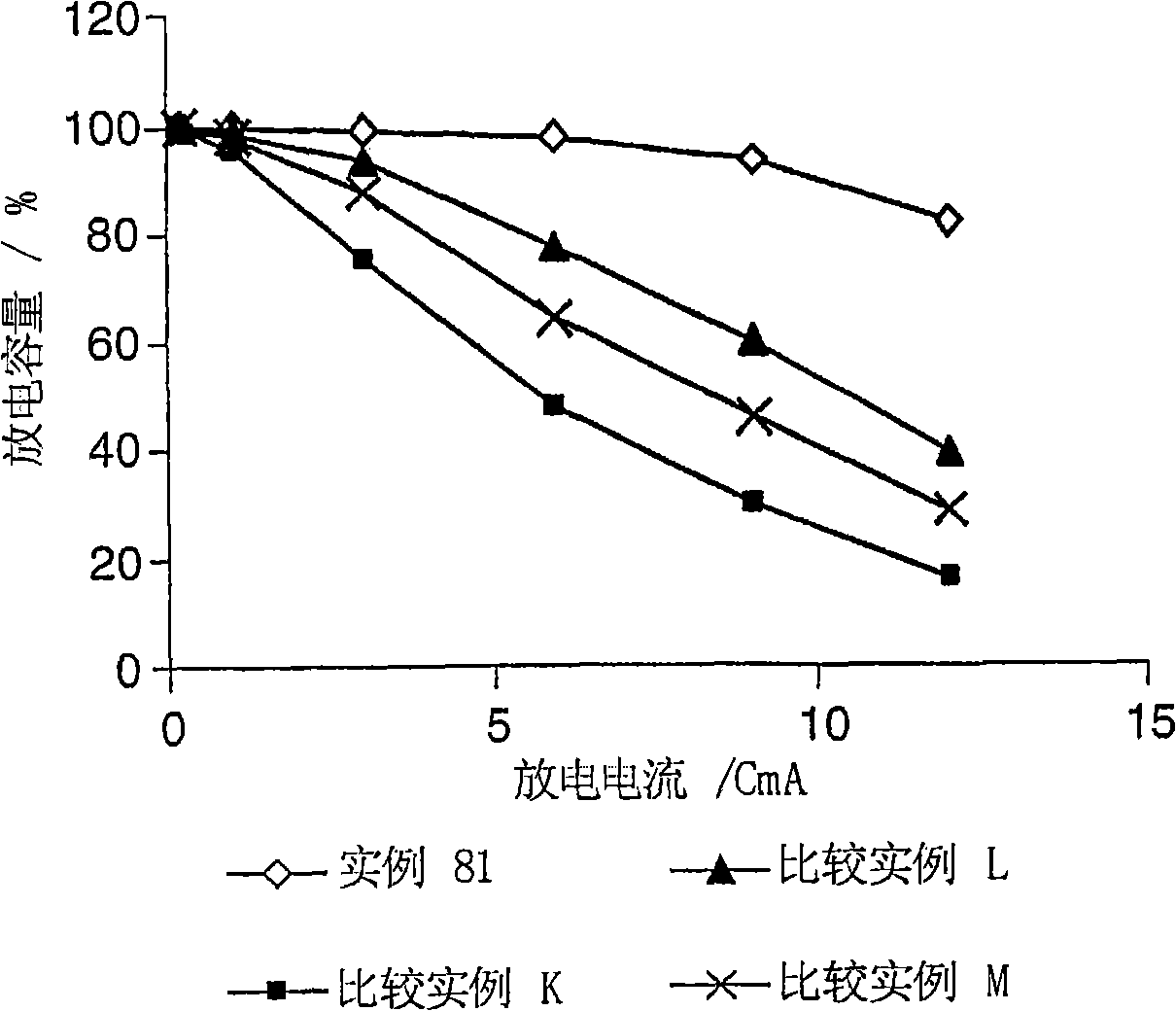
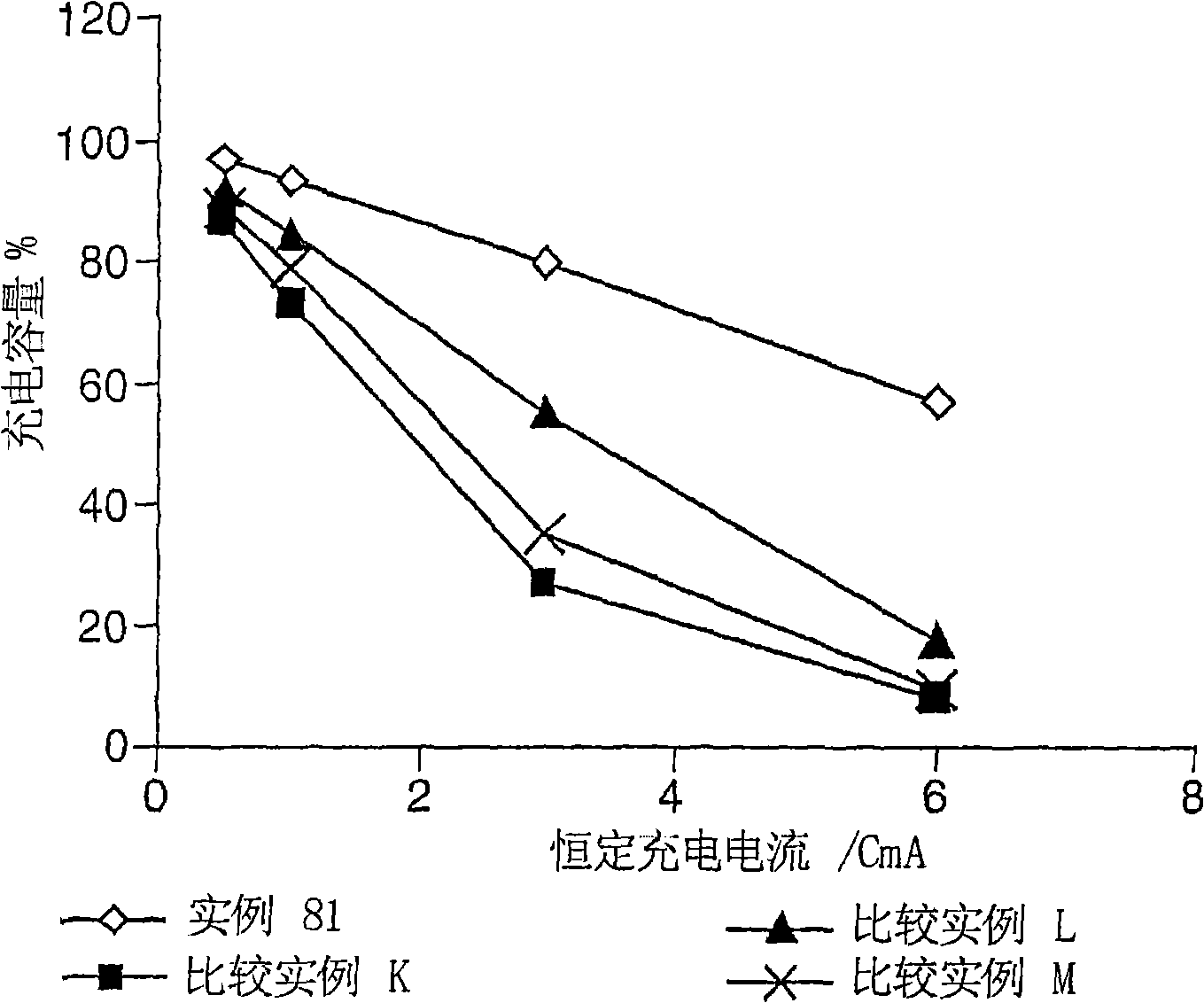
Electrolyte composition
A composition and solvent composition technology, applied in non-aqueous electrolyte storage batteries, circuits, lithium storage batteries, etc., can solve problems such as excessively high surface energy, inability to self-wet battery parts, and difficulty in volatile solvents.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] Preparation of hydrofluoroether compounds
[0052] Said hydrofluoroether compound is prepared by first carrying out a free radical addition reaction of at least one perfluoroalkene or perfluorovinyl ether starting compound with at least one hydrocarbon alcohol or an adductable fluorocarbon alcohol. This results in the formation of at least one fluoroalcohol intermediate. The fluoroalcohol intermediate can then be anionized with at least one perfluoroalkene or perfluorovinyl ether modifying compound (which may be the same or different than the perfluoroalkene or perfluorovinyl ether used in the first addition reaction) addition to form at least one hydrofluoroether compound. Alternatively, when the alcohol is polyfunctional, the type of addition reaction can be reversed, wherein the first addition reaction is anionic addition and the second addition reaction is free radical addition. Therefore, the sequence of steps is non-limiting and can be modified to obtain the d...
example
[0092] Objects and advantages of this invention are further illustrated by the following examples, but the particular materials and amounts thereof recited in these examples, as well as other conditions and details, should not be construed to unduly limit this invention. These examples are illustrative only, and are not intended to limit the scope of the appended claims.
[0093] All parts, percentages, ratios, etc. in the examples and in the rest of the specification are by weight unless otherwise indicated. Solvents and other reagents used were obtained from Aldrich Chemical Company, Milwaukee, Wisconsin unless otherwise indicated.
[0094] In the preparation of the following compounds, diastereomeric mixtures are obtained due to the presence of two (or more) optical centers in the molecule. These diastereomers have boiling points very close to each other and therefore diastereomers cannot be separated by distillation. In many cases, however, the above diastereomers are re...
Embodiment 1 to 65
[0162] Examples 1 to 65 were implemented to determine the stability of the electrolyte composition of the present invention. 1 mole lithium hexafluorophosphate (LiPF 6 ) is dissolved in 1 L of solvent mixture and then equilibrated at a predetermined temperature to prepare the sample. The solvent mixture comprises ethylene carbonate (EC), diethyl carbonate (DEC), ethyl methyl carbonate (EMC) and hydrofluoroether (HFE) according to the invention in different proportions. The HFE always used for instances 1 to 83 is CF 3 CFHCF 2 CH(CH 3 )OCF 2 CFHCF 3 Or compound No. 3 above. The stability of the electrolyte composition was determined by visual observation of the samples for clarity, homogeneity, and presence of multiple phases. A transparent, homogeneous and single-phase electrolyte composition at a given temperature leads to the conclusion that it is stable at this temperature. Table I below summarizes the results of the visual observations, the temperatures at which th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com