Sunscreen cosmetic composition
A technology of cosmetics and chemical formulas, applied in the direction of cosmetics, skin care preparations, etc., to achieve the effects of improved adhesion, excellent ultraviolet absorption performance, and excellent product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0157] The following examples illustrate the present invention more specifically, but the present invention is not limited by these examples.
[0158] [the first invention]
[0159]
Synthetic example 1-1
[0160] "Synthesis Example 1-1: Synthesis of 2-[4-(2-ethylhexyloxy)-2-hydroxyphenyl]-2H-benzotriazole"
[0161] 45.4g (0.2mol) of 6-(2H-benzotriazol-2-yl) resorcinol synthesized according to the conventional method was added in a 500ml four-necked flask equipped with a thermometer and a reflux condenser, and 50ml of methyliso Butyl ketone and 4.0 g of dimethylformamide were stirred. 25.4 g (0.24 mol) of sodium carbonate and 77.2 g (0.4 mol) of 2-ethylhexyl bromide were added thereto, and heated to reflux temperature while stirring. Stir for 15 hours while maintaining the reflux temperature, then recover methyl isobutyl ketone under normal pressure, wash the remaining oil with water, and remove excess sodium carbonate and generated inorganic substances. Vacuum distillation was carried out from this oil to obtain 52.1 g of a yellow transparent fraction at 220-225° C. / 0.2-0.3 mmHg. The compound is liquid at room temperature, the yield is 76.7%, and the HPLC purity is 99.0%.
Synthetic example 1-2
[0162] "Synthesis Example 1-2: Synthesis of 2-(2-Hydroxy-4-isobutoxyphenyl)-2H-benzotriazole"
[0163] Synthesis was carried out in the same manner as in Synthesis Example 1, using an equimolar amount of isobutyl bromide instead of 2-ethylhexyl bromide. Yellowish off-white powdery crystals were obtained with a yield of 72.5%. m.p.120.0~120.8°C, λmax=345.6nm, ε=21750.
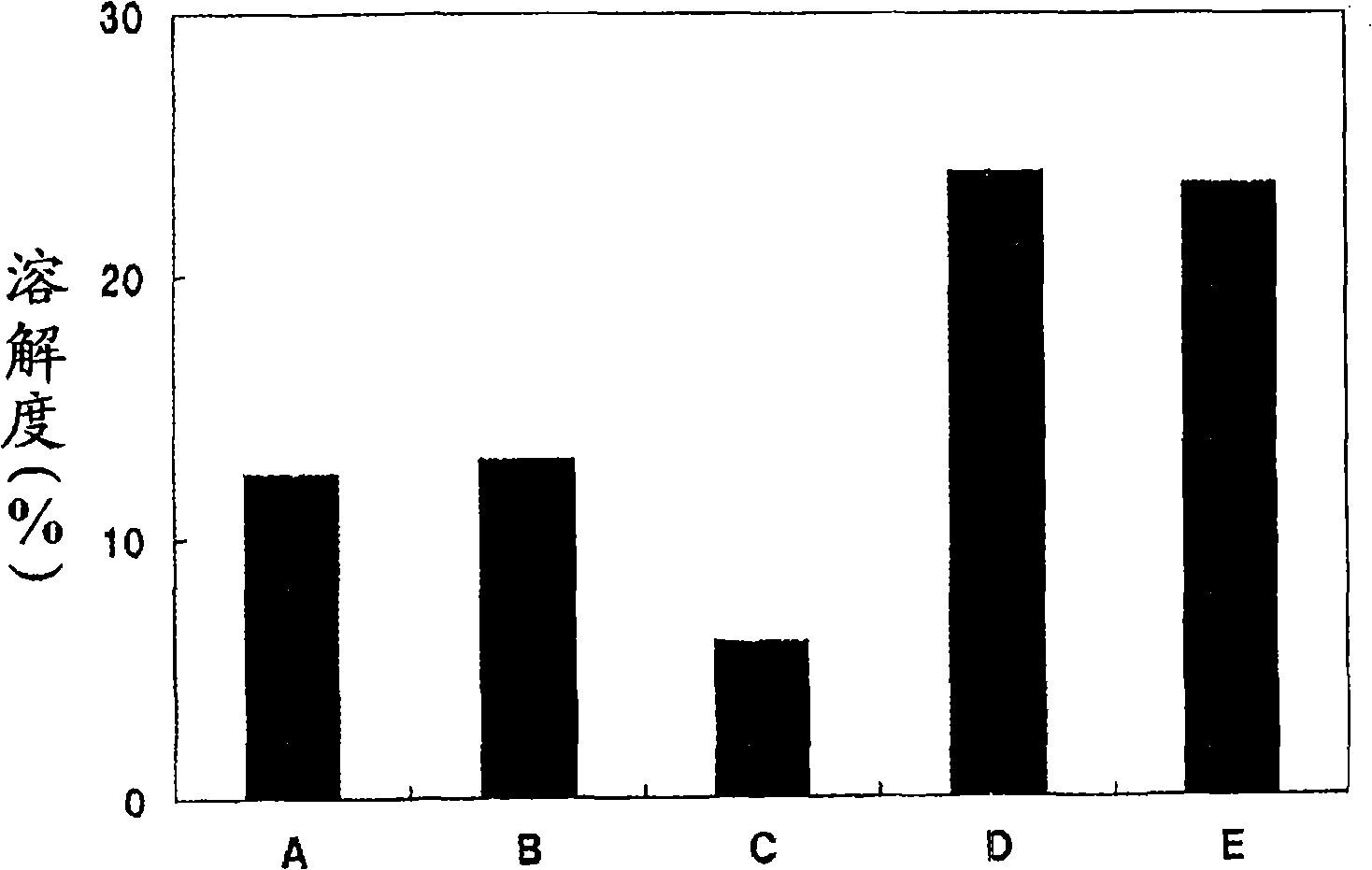
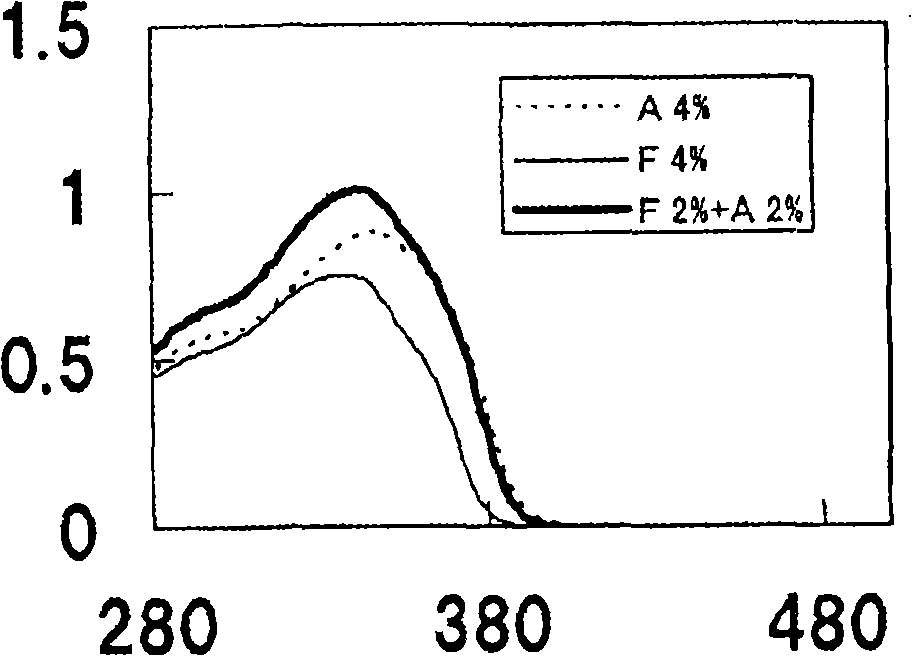
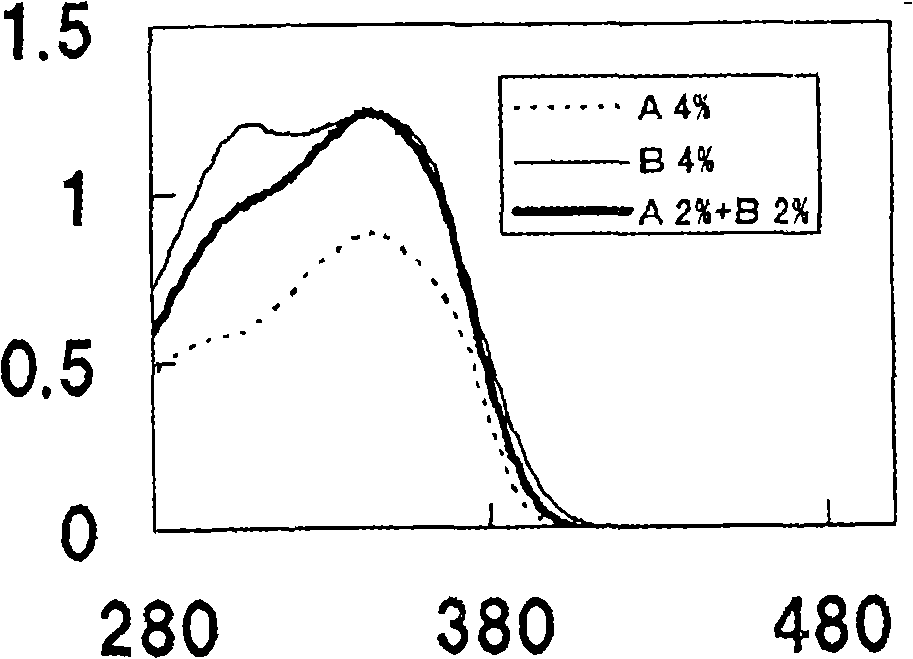
[0164] Research the oils of following A~E to 2,4-bis[[4-(2-ethylhexyloxy)-2-hydroxyl]phenyl]-6-(4-methoxyphenyl)-1, The solubility of 3,5-triazine, the results are as figure 1 shown.
[0165] A: Isodecyl Benzoate
[0166] B: 2-ethylhexyl benzoate
[0167] C: Ethylhexyl 2-cyano-3,3-diphenylacrylate (octocrylene)
[0168]
[0169] D: 2-[4-(2-Ethylhexyloxy)-2-hydroxyphenyl]-2H-benzotriazole
[0170]
[0171] E: 2-(2-Hydroxy-4-isobutoxyphenyl)-2H-benzotriazole
[0172]
[0173] "Solubility Determination Methods and Conditions"
[0174] 2,4-bis[[4-(2-ethylhexyloxy)-2-hydroxy]phenyl]-6-(4-methoxyphenyl)-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com